 |
 |
 |
| |
Decline in Renal Function Associated with Tenofovir DF (TDF) Compared to Nucleoside Reverse Transcriptase Inhibitor (NRTI) Treatment
|
| |
| |
Joel E. Gallant, Michelle Parish, Jeanne Keruly, Richard Moore
Johns Hopkins University School of Medicine, Baltimore, MD
Poster # N-138
12th Conference on Retroviruses
and Opportunistic Infections
Boston, MA
February 25, 2005
"...Over a median treatment duration of 322 days, TDF use was associated with a 4% greater decline in CLCr compared with alternative NRTIs..Renal function should be assessed prior to starting TDF, and TDF dosing interval should be adjusted in those with impaired renal function as recommended. Renal function should be monitored in TDF-treated patients.."
ABSTRACT
Background: Tenofovir DF (TDF) is a nucleotide analog reverse transcriptase
inhibitor used for the treatment of HIV disease. Despite demonstrated renal
safety in several clinical trials, case reports and observational data suggest that it may be associated with nephrotoxicity.
Methods: We analyzed data from a large prospective observational cohort,
comparing all patients who initiated TDF (N=344) or an alternative nucleoside
analog reverse transcriptase inhibitor (NRTI, N=314) after Jan. 1, 2001 and
who had at least two serum creatinines (Cr) drawn within 90 days of initiation.
We assessed change and percentage change in calculated Cr clearance (CLCr)
over the study period for each subject. Baseline CLCr was calculated using the
Cockcroft-Gault equation using the average of the 2 serum Cr measurements
obtained closest to the start of therapy.
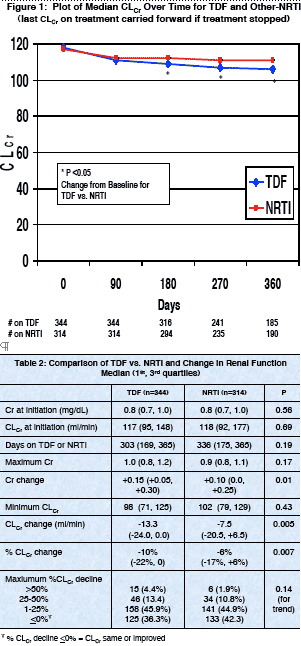
Results: TDF recipients had greater increases in Cr and greater absolute and percentage declines in CLCr compared to those in the NRTI group, although
there was no difference in rates of discontinuation coincident with maximum
decline in CLCr. Med. baseline Cr and CLCr were 0.8 mg/dL and 117.5 ml/min,
respectively, with no differences between groups. Median Cr change was +0.15
and +0.10 in the TDF and NRTI groups, respectively (p=0.01). Med. CLCr
change was -13.35 and -7.5 ml/min (p=0.005). The changes were apparent by
90 days of therapy and persisted over the entire year. The percentage change
in CLCr was also associated with longer duration of treatment with TDF or the
NRTI, diabetes, higher baseline Cr, and CD4 count <50 cells/mm3 (p <0.05). In
a multivariate analysis only TDF use and lower CD4 count were associated with
CLCr decline (p<0.05), with a trend toward association with lower baseline CLCr
and diabetes. In this analysis, hypertension, use of LPV/r or any other
concomitant antiretroviral agents, viral load, previous use of adefovir, age, sex, race and HIV transmission risk group were not associated with CLCr decline.
Discussion: Use of TDF is associated with a modest decline in CLCr, especially in patients with baseline renal insufficiency, diabetes, or low CD4 count. While
the differences were statistically significant, they were small in magnitude and
of unclear clinical significance. Patients at risk for renal dysfunction should be carefully monitored when taking TDF, and the dosing interval should be
adjusted when indicated by a reduced creatinine clearance.
BACKGROUND
⋅ TDF is renally excreted via a combination of glomerular filtration and active tubular secretion.
⋅ Adefovir dipivoxil, a related NtRTI, caused proximal renal tubular dysfunction at the daily doses of 60 to 120 mg required to inhibit HIV replication.
⋅ TDF has an excellent renal safety profile in clinical trials.
⋅ Renal impairment (including acute renal failure and Fanconi Syndrome) has been associated with TDF use in some cohort studies. Most cases have occurred in patients with underlying systemic or renal disease or in patients taking nephrotoxic agents.
⋅ Despite the apparent renal safety of TDF in placebo-controlled clinical trials, these reports raise concerns about nephrotoxicity.
⋅ We analyzed data from a large clinical database to assess the effect of TDF on renal function in clinical practice.
METHODS
⋅ Prospective observational database in Johns Hopkins HIV Clinical Cohort, which has enrolled over 5000 patients since 1990.
⋅ Analysis of data from all patients who initiated HAART with either TDF or any alternative NRTI after January 1, 2001 and who had at least 2 serum creatinines (Cr) drawn within 90 days of initiation.
⋅ Data included: age, race, sex, mode of HIV transmission, weight at
initiation, presence or history of diabetes and hypertension, Cr at start and
completion of study period, current ART regimen, and CD4 count and HIV
RNA at start of treatment.
Definitions
⋅ Creatinine clearance (CLCr) calculated using Cockcroft-Gault equation.
⋅ Average of 2 Cr values used to minimize regression to the mean.
⋅ Baseline creatinine and CLCr : Average of the 2 Cr obtained closest to start of treatment. Baseline CLCr calculated using this average.
⋅ Change in Cr and CLCr: Calculated based on average of maximum Cr for each subject within 1 year of initiation while on treatment and next Cr. If no on-treatment Cr measurement after maximum Cr, then maximum Cr and Cr immediately prior to maximum were averaged.
Analysis
⋅ Change and % change in CLCr for each subject over study period
⋅ Statistical significance of change in CLCr for patients treated with TDF vs. those treated with other NRTIs.
⋅ Statistical analysis: Wilcoxon rank-sum test (TDF vs. NRTIs and other bivariate comparisons), and multivariate general linear modeling (associations of multiple factors with CLCr change).
RESULTS
| |
| |
| |
 |
|
| |
| |
 |
|
| |
| |
RESULTS (Continued)
⋅ It is now recommended that the dosing interval of TDF be modified in patients with a CLCr <50mL/min. Only 2 subjects, who had a baseline Cr >2.0 mg/dL, received less than 300 mg/day of TDF.
⋅ 19 (5.5%) of TDF- and 21 (6.7%) of NRTI-treated patients discontinued therapy at time of maximum decline in renal function (p>0.05).
Univariate associations: %CLCr change also associated with:
-diabetes (%CLCr change -13) vs. no diabetes (-8) [p<0.04]
-baseline CD4 <50 cells/mm3 (-14) vs. >50 (-8) [p<0.01]
-baseline HIV RNA>20,000 c/ml (-15) vs. >20,000 (-8) [p<0.02]
-initial therapy (-11) vs. subsequent therapy (-8) [p<0.03]
-Not associated: hypertension, age, gender, race, hepatitis C or B, use
of LPV/r or any other specific PI or NNRTI.
Multivariate analysis:
-Overall: only TDF use (p=0.006) and CD4 count <50 cells/mm3 (p<0.001) were associated with CLCr decline. Trends toward associations with baseline CLCr <50 ml/min and diabetes (p=0.10).
-Patients with baseline CLCr >50 ml/min: Associations unchanged: TDF use (p=0.004), low CD4 count (p<0.001); diabetes (trend, p=0.06)
-Not associated: hypertension, use of LPV/r or other ARVs, HIV RNA, prior use of adefovir, age, sex, race and HIV transmission risk group
CONCLUSIONS
⋅ Over a median treatment duration of 322 days, TDF use was associated with a 4% greater decline in CLCr compared with alternative NRTIs.
⋅ Advanced immunosuppression (CD4<50) was also associated with decline in renal function.
⋅ There were trends toward associations with diabetes and baseline CLCr <50 ml/min.
⋅ Restricting analysis to subjects with baseline CLCr >50, who should receive full-dose TDF, did not change the findings.
⋅ There was no association with age, race, sex, HIV transmission group, HTN, HIV RNA, use of LPV/r or other specific ARV agents, or prior use of adefovir.
⋅ Although statistically significant, the clinical significance of these findings is unclear. The decline in CLCr associated with TDF use was small and was not associated with greater rates of discontinuation.
⋅ The majority of TDF-treated subjects remain on TDF and will continue to be followed.
⋅ Renal function should be assessed prior to starting TDF, and TDF dosing interval should be adjusted in those with impaired renal function as recommended. Renal function should be monitored in TDF-treated patients.
| |
| |
| |
|
 |
 |
|
|