 |
 |
 |
| |
Reyataz/r: 96 week Study 045 results
|
| |
| |
Reported by Jules Levin
"The Influence of Baseline Protease Inhibitor (PI) Mutations on the Efficacy of Ritonavir-Boosted Atazanavir (ATV/r), Atazanavir Plus Saquinavir, and Lopinavir/Ritonavir (LPV/r) In Patients Who Have Experienced Virologic Failure on Multiple HAART Regimens"
Margaret Johnson reported this poster #711 at the 12th CROI with 96-week results from study 045, which compared Reyataz (atazanavir/r to Kaletra (lopinavir.r).
M Johnson1, E DeJesus2, C Rodriguez3, L Nieto-Cisneros4, A Rightmire5, L Odeshoo5, C McLaren5
1Royal Free Hospital, London, England; 2IDC Research Initiative, Altamonte Springs, FL; 3Hospital Argerich, Buenos Aires, Argentina; 4Hospital Gabriel Mancera, IMSS, Mexico City, Mexico; 5 Bristol-Myers Squibb, Wallingford, CT
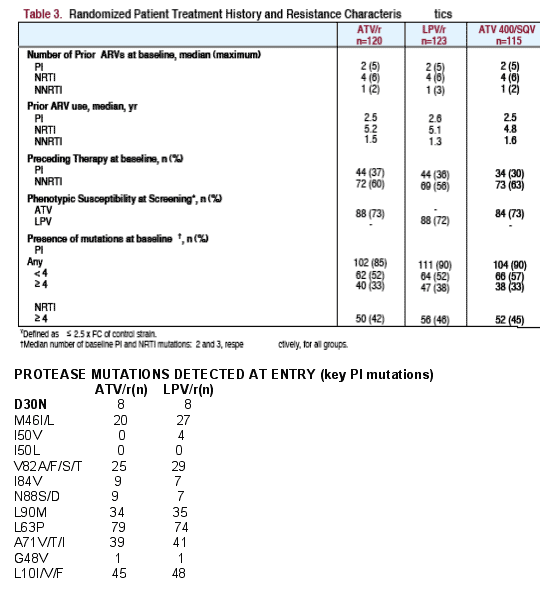
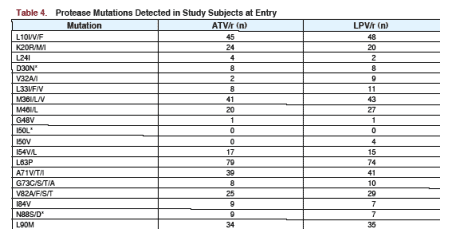
Johnson reported the present analysis was performed to assess the influence of baseline PI mutations on the virologic response to ATV- and LPV/r- containing regimens through 96 weeks. This study was a non-inferiority trial in treatment-experienced patients, examining to see if one regimen was inferior or not to the other. The resistance analysis reported in this study reported the viral load response for patients receiving ATV/r or LPV/r who had 4 or more PI mutations at baseline; as well as for patients with 4 or less PI mutations at baseline. There is a table below that lists the specific mutations patients had at baseline.
SUMMARY:
PRIOR ART median experience of patients: 2.5 years PIs; 5.2 yrs NRTIs; 1.4 yrs NNRTIs. Patients previously took average of 2 PIs, 4 NRTIs, 1 NNRTI. About 33% of patients receiving ATV/r & 38% receiving LPV/r had 4 or more PI mutations before entering the study. 42% of patients receiving ATV/r & 46% receiving LPV/r had 4 or more NRTI mutations before entering the study. The actual number of patients with specific mutations are listed below.
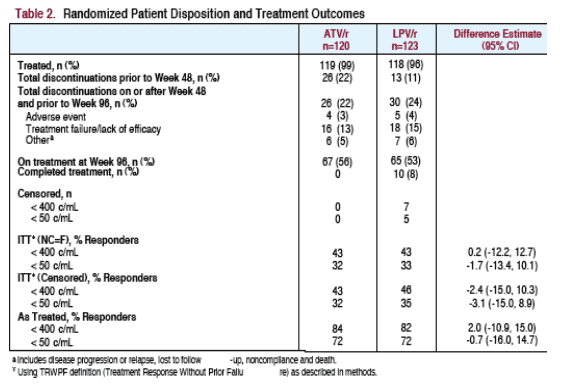
The viral load responses & proportion of patients with undetectable viral load at week 96 was similar for patients taking ATV/r or LPV/r. 56% of ATV/r and 53% of LPV/r patients remained on study through Week 96.
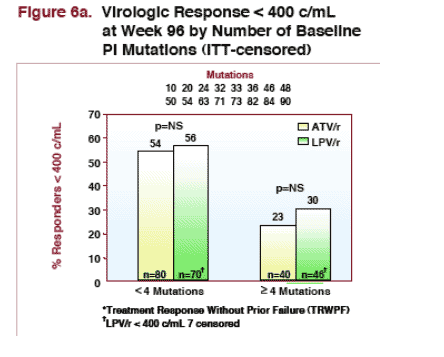
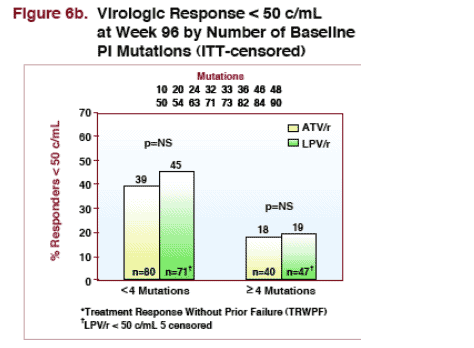
--for patients with 4 or more PI mutations at baseline: 23% taking ATV/r & 30% taking LPV/r had <400 copies/ml (NS) at week 96. 18% of patients taking ATV/r & 19% taking LPV/r had <50 copies/ml at week 96.
ITT (censored, % Responders)
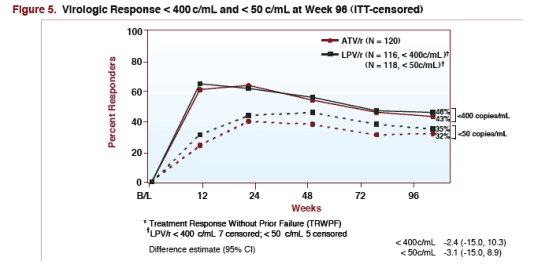
--46% taking LPV/r & 43% taking ATV/r had <400 copies/ml at week 96*
--35% taking LPV/r & 32% taking ATV/r had <50 copies/ml at week 96**
The ITT (NC=F) % Responders
<400 copies/ml: 43% for both groups ATV/r & LPV/r at week 96
<50 copies/ml: 32% for ATV/r & 33% for LPV/r at week 96
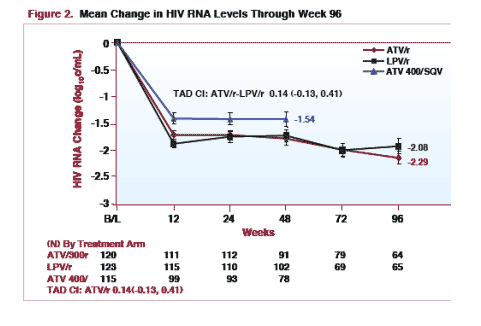
MEAN CHANGE in HIV RNA through week 96: -2.08 log (n=64) for LPV/r & -2.29 log (n=65) for ATV/r. There were 120 patients at baseline in each arm.
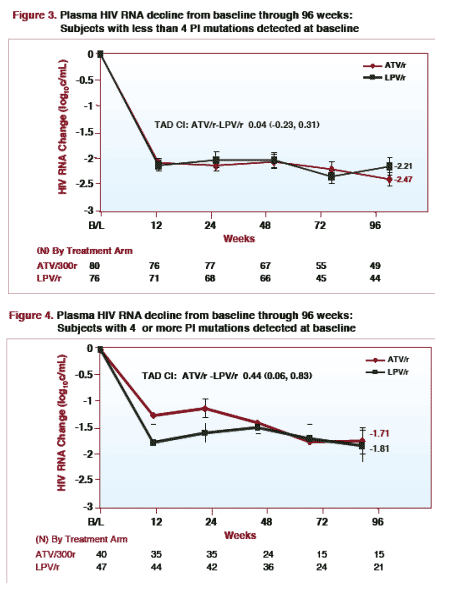
For patients with 4 or more PI mutations detected at baseline, plasma HIV RNA decline from baseline through week 96 was -1.71 log for ATV/r (n=15) & -1.81 log for LPV/r (n=21). [TAD CI: ATV/r ŠLPV/r 0.44 (0.06, 0.83)]
For patients with less than 4 PI mutations detected at baseline, plasma HIV RNA decline from baseline through 96 weeks was -2.21 log for LPV/r (n=44) & -2.47 log for ATV/r (n=49). [TAD CI: ATV/r-LPV/r 0.04 (-0.23, 0.31)]
AUTHOR CONCLUSIONS:
Once-daily ATV/r demonstrated efficacy similar to LPV/r twice-daily through 96 weeks in treatment-experienced patients.
Sub-set assessments of virologic response showed comparable efficacy between the two regimens for the sub-groups of subjects with < 4 and ≥ 4 PI mutations.
The magnitude of response for both regimens was inversely related to the number of baseline PI mutations, with greater mean reduction in HIV RNA in patients with < 4 PI mutations compared with those with ≥ 4 PI mutations.
AUTHOR SUMMARY:
Through 96 weeks, comparable overall efficacy of ATV/r and LPV/r was observed, as assessed by the primary endpoint, TAD (time-averaged difference in viral load), thus supporting the durability of the antiviral efficacy, previously demonstrated at Week 48:
--The ATV/r and LPV/r regimens were comparable in terms of number and frequency of PI mutations at baseline
--No significant difference was seen in the secondary measurements of efficacy between the ATV/r and the LPV/r regimens through 96 weeks assessed by percent of subjects < 50 copies/mL, percent of subjects < 400 copies/mL in an ITT analysis:
56% of ATV/r and 53% of LPV/r patients remained on study through Week 96
72% of patients on both arms had HIV RNA < 50 c/mL (on-treatment analysis)
In this treatment-experienced cohort, median exposure to antiretrovirals was 129-136 weeks on PIs, 251-269 weeks on NRTIs and 69-84 weeks on NNRTIs.
The decline in plasma HIV RNA from baseline through 96 weeks was similar in subjects treated with ATV/r or LPV/r who had less than 4 protease inhibitor resistance mutations detected at baseline.
Responses were also similar in subjects with 4 or more protease inhibitor resistance mutations detected at baseline. A potential limitation is that there were relatively fewer patients with ≥ 4 PI mutations.
Mean time on study therapy was approximately 76 weeks for both the ATV/ and the LPV/r treatment regimens. Overall, discontinuation of study therapy during the period from Week 48 to Week 96 was comparable between treatment regimens.
Background
Atazanavir is a potent, well-tolerated, once daily PI that has been extensively studied, including Phase III clinical trials of naïve patients versus a standard of
care regimen containing EFV and treatment experienced patients versus a standard of care regimens containing LPV/r.
ATV/r has demonstrated comparable efficacy in treatment experienced patients to the standard of care regimen containing LPV/r.
BMS AI424-045 was undertaken to evaluate the efficacy and safety of ATV/r and a dual PI combination of ATV and SQV, in comparison with LPV/r, each
co-administered with tenofovir (TDF) and 1 NRTI in treatment-experienced patients who had failed two or more prior HAART regimens that included one or
more NRTI, NNRTI and PI. Overall efficacy and safety results at weeks 24, 48 and 96 have been previously presented.
Resistance to ATV is associated with the unique I50L substitution, which is found in clinical isolates from some subjects (primarily previously treatment-naive)
experiencing virologic failure during ATV-containing therapy.
HIV isolates typically show increasingly broad resistance to marketed PIs with an increase in the number of PI-associated mutations. The present analysis
was performed to assess the influence of baseline PI mutations on the virologic response to ATV- and LPV/r- containing regimens through 96 weeks.
Objectives Primary
Antiviral efficacy assessed by the time-averaged difference (TAD) from baseline in viral load (log10) between each of 2 ATV-containing regimens and a
LPV/r-containing regimen at Week 96.
Secondary
To assess the proportion of subjects with HIV RNA levels less than the limit of quantification ([LOQ] equals 400 c/Ml and LOQ equals 50 c/mL) through
Week 96
To assess the magnitude of changes in CD4 cell counts through Week 96
Additional secondary objectives include the assessment of serum lipids, safety and tolerability
Sub-set Analysis by Baseline PI Mutations
Describe the antiviral efficacy, assessed by change in HIV RNA, treatment response rates below LOQ 400 c/mL and 50 c/mL using ITT analyses based
upon number of baseline PI mutations (≥ or <4)
Methods
Multinational, open-label, 3-arm, randomized Phase III study in treatment-experienced patients with virologic failure on ≥ 2 HAART regimens that included,
in total, at least 1 PI, NRTI, and NNRTI
Primary Efficacy Analysis
Similarity (noninferiority) of efficacy between the ATV/r regimen and the LPV/r regimen was based on an upper 97.5% confidence interval (CI) for the TAD
estimate of <0.5 log10 copies/mL
Interim analysis at 24 weeks found the ATV/SQV arm to be inferior to both ATV/r and LPV/r arms. Patients were given the option of changing therapy and
therefore analysis of this arm was not continued past 48 weeks.
The study was subsequently extended and is currently continuing past Week 96.
Secondary Efficacy Analysis
ITT (NC=F) response rates include all randomized patients and count as responders patients with a minimum of two sequential HIV RNA measurements
< 400 c/mL (or < 50 c/mL) maintained through week 96
ITT (censored): patients who completed the study at week 48 while in response were removed from the analysis at the point of study completion (in speaking with BMS this analysis does not include the 10 patients on LPV/r referred to in Table 2 who completed 48 weeks & discontinued the study).
Sub-set Analysis of Efficacy by Baseline PI Mutations
Resistance mutations were selected using the Stanford Panel
Efficacy was assessed using TAD for HIV RNA levels, ITT response rates were conducted as described above
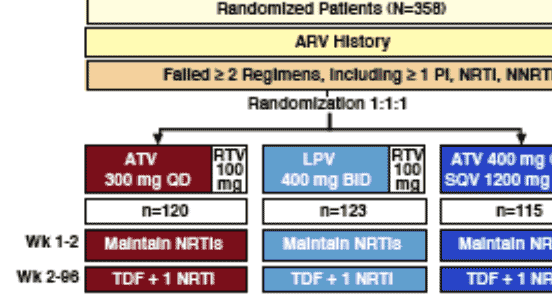
STUDY DESIGN
| |
| |
| |
 |
|
| |
| |
BASELINE CHARACTERISTICS
Baseline median viral load was 4.42 to 4.47 in all 3 treatment arms, about 26,000 copies/ml. 23-27% of the patients had HIV RNA <100,000 c/ml at baseline. Median CD4 counts were 283-317. 29-31% of the patients had <200 CD4s at baseline. About 60% were white, 23% Hispanic/latino, 15% black. 20-23% were female. 16-18% had HBV or HCV. 29% had AIDS. Median age was 39-41 yrs.
TREATMENT OUTCOMES & PATIENT DISPOSITION
| |
| |
| |
 |
|
| |
| |
VIROLOGIC RESPONSE <400 & <50 c/ml at Week 96 (ITT-Censored)
TRWPF: treatment response without prior failure
*LPV/r <400 c/ml: 7 censored; <50 c/ml: 5 censored
<400 c/ml
--46% LPV/r (n=116)*
--43% ATV/r (n=120)
<50 c/ml
--35% LPV/r (n=118)*
--32% ATV/r
| |
| |
| |
 |
|
| |
| |
 |
|
| |
| |
 |
|
| |
| |
TREATMENT HISTORY & RESISTANCE CHARACTERISTICS
--Patients had median prior 2 PIs in ATV/r & LPV/r arms; 4 NRTIs in each arm; 1 NNRTI in each arm.
--Median prior use by years: PIs, 2.5-2.6 yrs; NRTIs, 5.1-5.2 yrs; NNRTIs, 1.3-1.5 yrs.
--at screening phenotypic susceptibility was 73% to ATV/r & 72% to LPV/r for the patients receiving that treatment (defined as <2.5 fold x fold change of control strain.
--presence of mutations at baseline:
PI
Any: 85% in ATV/r arm & 90% in LPV/r arm
<4 muations: 52% in each artm
4 or more mutations: 33% in ATV/r & 38% in LPV/r arm.
NRTI
4 or more mutations: 42% in ATV/r & 46% in LPV/r arm
| |
| |
| |
 |
|
| |
| |
 |
|
| |
| |
 |
|
| |
| |
 |
|
| |
| |
|
 |
 |
|
|