 |
 |
 |
| |
Improved Lipids After Switch to Reyataz
|
| |
| |
Reported by Jules Levin
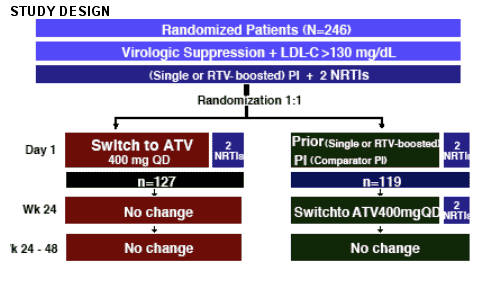
Two poster studies were reported at the 12th CROI in which patients with elevated lipids switched from another HAART regimen to atazanavir (Reyataz). Both studies showed significantly improved lipids after switching. One study examined Spanish patients in the Atazanavir Expanded Access Program, which allowed patients access to atazanavir if they had elevated lipids. This analysis examined patients who switched to ritonavir boosted atazanavir (300/100). The results report improvements in lipids. I'll report the results after I report the results from a prospective study. This study was designed to examine the effect on lipid parameters of a switch to ATV 400 mg QD from another PI (boosted or not) in a virologically suppressed, treatment-experienced patient population.
Plasma concentration of Apolipoprotein B and Lipoprotein a are strongest predictors of cardiovascular risk events. This is the first systematic evaluation of
the changes associated when switching to ATV. This study examined changes in lipids from baseline through week 12 after switching, and looked these lipid parameters:
--fasting LDL cholesterol
--fasting total cholesterol
--fasting HDL cholesterol
--fasting ApoB
--fasting LP(a)
--fasting triglycerides
Michael Sension reported these study results in poster #858 at the 12th CROI.
| |
| |
| |
 |
|
| |
| |
use of TDF was not allowed due to the PK interaction with ATV. Use of lipid lowering therapy within 4 weeks of study or during the study prohibited. Changes in nukes prohibited.
SUMMARY:
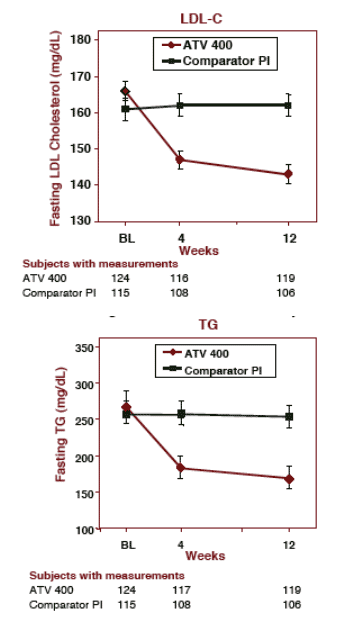
LIPIDS IMPROVED after 12 weeks for patients who switched to ATV 400 (mean values):
total cholesterol improved from 254 to 211 mg/dL (12.9%)
LDL-chol improved from: 166 to 143 mg/dL (13.8%)
Triglycerides improved from: 267 to 168 mg/dL (37%)
HDL cholesterol: improved from 44 to 46 mg/dL
Patients who remained on their current PI regimen did not have any changes in lipids during the 12 weeks.
Patients staying on comparator PI (mean values):
Total chol:
Baseline- 248
Week 12- 249
LDL-C:
Baseline- 161
Week 12- 162
TG:
Baseline- 257
Week 12: 254
HDL-chol:
Baseline- 41
Week 12- 42
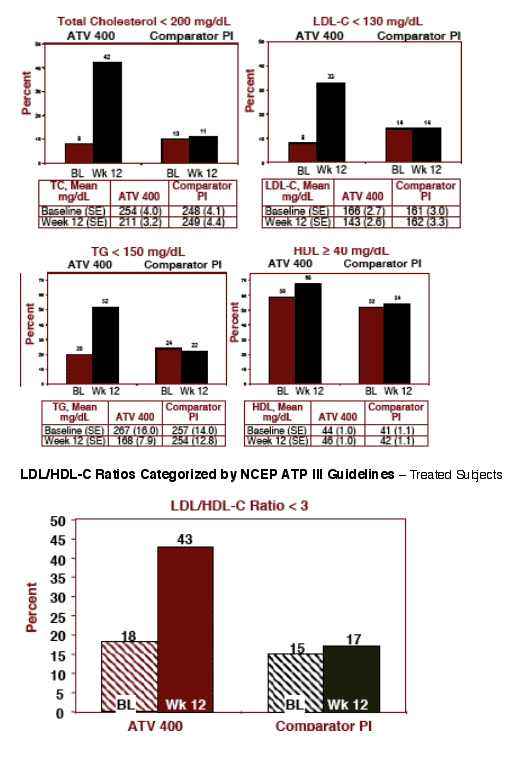
FASTING LDL-C: at baseline 8% of patients who switched to ATV 400 had <130 mg/dL (NCEP Guidelines for normal LDL), at week 12 this improved to 33% of patients who had <130 mg/dL LDL-C.
TOTAL CHOLESTEROL: at baseline 8% of patients who switched to ATV 400 had <200 total chol (NCEP Guidelines for normal cholesterol), at week 12 42% of patients on ATV 400 had <200 cholesterol.
TRIGLYCERIDES: at baseline 20% of patients who switched to ATV 400 had <150mg/dL (NCEP Guidelines for normal TG), at week 12 52% of patients taking ATV 400 had <150 mg/dL TG.
HDL CHOLESTEROL: at baseline 59% who switched to ATV 400 had 40 or more mg/dL (NCEP Guidelines), at week 12 68% of patients who switched to ATV 400 had improved to above 40.
The percentages of patients remaining on the comparator PI with normal lipid levels by NCEP Guidelines did not change from baseline to week 12.
See table below showing changes in all lipid parameters followed in this study.
EFFICACY
No differences in efficacy between arms were observed through week 12. Full assessment of antiviral efficacy end-points will be completed at 48 weeks
of follow up.
Of the 209 patients on treatment with HIV RNA < 50 c/mL at baseline, 2 patients in the ATV-arm (2%) and 1 in the Comparator PI arm (1%) had viral rebound > 400 copies/ml through week 12
The proportion of patients discontinuing due to lack of efficacy was the same for each treatment group (<1%)
Mean increases from baseline in CD4 cell counts were comparable between treatment arms, although smaller increases were observed in ATV arm
"AI424067: Improvement in Lipid Profiles After 12 Weeks of Switching to Atazanavir (ATV) From Boosted or Unboosted Protease Inhibitors (PIs) in Patients With No Previous PI Virologic Failure and Hyperlipidemia at Baseline"
M Sension1, B Grinsztejn2, JM Molina3, I Zavala4, F Antunes5, A Donnelly6, P Agarwal6, and E Ledesma6
1Comprehensive Care Center, Fort Lauderdale, Florida; 2Hospital Evandro Chagas, Rio de Janeiro, Brazil;
3Hospital Saint-Louis, Paris, France; 4Hospital Dr. Angel Leano Consultorio de Infectiologica, Jalisco, Mexico;
5Hospital Santa Maria, Lisbon, Portugal, 6Bristol-Myers Squibb, Wallingford, Connecticut
Background
--Atazanavir is a potent, well-tolerated, once daily PI that has been extensively studied, including Phase III clinical trials of naive patients versus a standard
of care regimen containing EFV and treatment experienced patients versus a standard of care regimen containing LPV/r.
--Elevated plasma lipid levels are associated with increased cardiovascular risk. The National Cholesterol Education Program (NCEP) Adult Treatment
Panel III (ATP III) guidelines recommend pharmacologic intervention when fasting LDL-C values are >130 mg/dL to mitigate the risk of cardiovascular
complications.
--Studies have demonstrated that ATV, RTV-boosted or not, in contrast to other currently approved PIs, does not lead to increases in total cholesterol (TC),
low-density lipoprotein cholesterol (LDL-C) and/or triglycerides (TG).
--DHHS Guidelines for the treatment of adults with HIV infection include switching to ATV as an option for the management of the increased cardiovascular risk likely associated with all other PIs.
--Prior exploratory studies (AI424044, AI424043) suggested that a switch to ATV led to improved lipid levels as early as 12 weeks of follow-up, and that the
improvement was maintained through at least 48 weeks.
--This study was designed to examine the effect on lipid parameters of a switch to ATV 400 mg QD from another PI (boosted or not) in a virologically
suppressed, treatment-experienced patient population.
--Plasma concentration of Apolipoprotein B and Lipoprotein a are strongest predictors of cardiovascular risk events. This is the first systematic evaluation of the changes associated when switching to ATV.
Objectives
Primary
Compare the week 12 percent (%) change from baseline in fasting LDL-C between patients on an ATV-containing ARV-regimen and those remaining on a
comparator PI-regimen
Secondary
To evaluate:
--Changes from baseline at week 12 in fasting TC, HDL cholesterol (HDL-C), fasting TG, non-HDL cholesterol, Apolipoprotein B (ApoB), Lipoprotein a (Lpa)
--Time to virologic rebound
--Changes from baseline in CD4 cell counts through week 12
--Changes from baseline in serum fasting glucose and insulin levels at week 12
Safety and tolerability of ATV
METHODS
Study Patients
--HIV-1-infected men and women ≥ 16 years of age
--On stable PI-containing ARV regimen (with or without RTV-boosting) for a period ≥ 3 months
--HIV RNA <50 copies/ml at screening
--No known history of virological rebound while on PI therapy
--Fasting LDL-C > 130 mg/dl at screening
Evaluations
Safety
Through week 12:
--Mean % changes from baseline in fasting LDL-C, TC, HDL-C, non-HDL-C, ApoB, Lp(a) and TG
--Directly measured LDL-C
-- Median and mean changes from baseline in fasting glucose and insulin
- All observations available through week 24:
Frequency and severity of all clinical and laboratory adverse events, and discontinuations for adverse events.
Efficacy (through week 12)
- Time to virological rebound (2 consecutive HIV RNA levels ≥ 400 copies/ml)
- Hazard ratio for subjects with HIV RNA <50 copies/ml at baseline
- Magnitude and durability of increases from baseline of CD4 cell counts in terms of time-averaged difference (TAD)
Statistical Analyses
- 90% power to detect a difference of ≥ 14% between the immediate and delayed switch groups in Week 12 mean % change from baseline in fasting LDL-C
- Primary analyses included the assessment of the mean percent change from baseline in fasting LDL-C at Week 12. The ATV (immediate switch) regimen declared to be superior to the comparator PI (delayed switch) if UL 95% CI for difference < 0
Results
Prior ARV Therapy, Study Drug Exposure, Disposition and Demography results
- Mean time on PI, NNRTI and NRTI prior to baseline: 184, 74, and 220 weeks, respectively
- 39% of patients were on a RTV-boosted PI-containing regimen
- Mean time on study regimen:
ATV: 22 weeks
PI-comparator: 19 weeks
Subject disposition (see Table 1)
MEAN FASTING LDL CHOLESTEROL & TG From Baseline Through Week 12
- Treated Subjects
| |
| |
| |
 |
|
| |
| |
Difference Estimates of Lipids Change from Baseline Through Week 12 - treated Subjects (observed values; primary endpoint: fasting LDL cholesterol)
This table reports the difference in change in lipids through week 12 between the patients receiving ATV vs the patients remaining on their current regimen.
Percent Change from Baseline
ATV-Comparator PI (p-value)
Fasting LDL-C (mg/dL) -15% (-19.1%, -10.8%) <0.0001
Fasting total chol -17.4% )-20.3%, -14.4%) <0.0001
Fasting HDL-C 5.1% (-0.1%, 10.6%) 0.057
Fasting non-HDL-C -22.1% (-25.5%, -18.5%) <0.0001
Fasting ApoB -19.5% (-22.9%, -15.9%) <0.0001
Fasting LP(a) -21.1% (-31.6%, -8.9%) 0.0014
Fasting triglycerides -34.6% (-41.8%, -26.5%) <0.0001
Lipid Parameters by NCEP-ATP III Criteria
Based on the NCEP-ATP III criteria, the lipid profile of the subjects at week 12 in the ATV arm was more favorable than that of the patients in the comparator PI arm (see Figures 3a and 3b):
--More patients in the ATV arm had a fasting LDL cholesterol level of < 130 mg/dL (33% vs 14%). Patients remaining on comparator PI had no change while % of patients on ATV 400 with <200 LDL-C increased from 8% to 33%
- More patients in the ATV arm had Total Chol. levels < 200mg/dL (42% vs 11%) and triglycerides levels of < 150 mg/dL (52% vs 22%). Again, patients remaining on comparator PI showed no change while the % of patients on ATV with <130 LDL & <150 TG increased
- More patients in the ATV arm had HDL cholesterol levels of ≥ 40 mg/dL (68% vs 54%)
- Patients in the ATV arm experienced improvement in NCEP categories from 8% at baseline to 42% at week 12 for TC < 200 mg/dL, for LDL-C < 130
mg/dL from 8% to 33 %, for TG < 150 mg/dL from 20% to 52% and for HDL-C ≥ 40 mg/dL from 59% at baseline to 68% at week 12
--More patients experienced an improvement in the ATV arm in LDL/HDL-C ratio of less than 3 (43% vs 17%)
Lipid Levels Categorized by NCEP ATP III Guidelines - Treated Subjects
| |
| |
| |
 |
|
| |
| |
Efficacy
No differences in efficacy between arms were observed through week 12. Full assessment of antiviral efficacy end-points will be completed at 48 weeks
of follow up.
Of the 209 patients on treatment with HIV RNA < 50 c/mL at baseline, 2 patients in the ATV-arm (2%) and 1 in the Comparator PI arm (1%) had viral
rebound > 400 copies/ml through week 12
The proportion of patients discontinuing due to lack of efficacy was the same for each treatment group (<1%)
Mean increases from baseline in CD4 cell counts were comparable between treatment arms, although smaller increases were observed in ATV arm
Deaths, Adverse Events, and Laboratory Abnormalities Through Week 24
--Discontinuations due to AEs were low and comparable for both groups [ATV=3(2%), comparator PI=1(<1%)]
--TOTAL BILIRUBIN ELEVATION: 22% (27/124) in the ATV arm & 1% in the comparator arm.
Serious adverse events: 2% in ATV, 3% in comparator PI
Grade 3-4 Lab Abnormalities:
Neutrophil reduction: 2% ATV, 0% comparator PI
ALT elevation: <1% ATV, 3% comparator PI
AST elevation: <1% ATV, <1% comparator PI
5% incidence of jaundice & 2% ocular icterus for any grade reported in ATV arm; 1 subject had grade 3-4 jaundice or ocular icterus
BASELINE CHARACTERISTICS & PATIENT DISPOSITION
Median HIV RNA: 1.69 log
Weeks on prior PI: 183
Weeks on prior NRTI: 217-222
Weeks on prior NNRTI: 68-79\baseline CD$ count: 463-470
Age: 42-44
80% male
71-77% white; 11-14% latino; 10-15% black.
| |
| |
| |
| |
|
 |
 |
|
|