 |
 |
 |
| |
Kaletra Independently Reduces HIV Replication in Cerebrospinal Fluid
|
| |
| |
12th CROI abstract 411.
Authors and Affiliations: G van den Brande1, J Marquie-Beck1, E Capparelli1, R Ellis1, A McCutchan1, A Hermes2, M Buzzell1, and Scott Letendre*1
1Univ of California, San Diego, USA and 2Abbott Labs, Abbott Park, IL, USA
Study was supported by Abbott & the National Institute of Mental Health
--Kaletra (LPV/r) monotherapy was administerd to treatment-naïve individuals for 3 weeks
--This study reports that LPV/r effectively reduced HIV replication in CSF at rates similar to plasma in individuals with higher CD4 counts & higher HIV RNA levels in CSF.
--Kaletra reduced HIV RNA in CSF in individuals, and in those more advanced HIV disease. Slower declines probably reflect HIV replication in longer-lived CNS cells and are consistent with prior findings using combination ARV therapy, see table below demonstrating slow & faster HIV RNA decline in the CSF during the initial 3 weeks therapy with Kaletra monotherapy. Importantly, two of the subjects who had slower declines of HIV RNA in CSF suppressed below 1.7 log c/ml with LPV/r alone.
--LPV/r-containing combinations initiated after the first 3 weeks of Kaletra monotherapy durably suppress HIV RNA in CSF in this study. HIV RNA levels were below 1.7 log copies/ml in all subjects at weeks 12 and 24.
--the study authors reflect that sequential introduction is a safe method for determining the effectiveness in the CNS of ARTs that have a high barrier to resistance.
--And they conclude that LPV/r benefits individuals at risk for or diagnosed with HIV-associated neurocognitive impairment.
Study investigators administered LPV/r (Kaletra) to ARV-naïve individuals for 3 weeks before starting other ARVs. The primary study objective was to determine whether LPV/r reduces HIV RNA levels in CSF when used by itself. Of the 16 subjects, 12 were enrolled and were started on LPV/r alone for 3 weeks. At least 2 other ARV were then added to complete 24 weeks. CSF was obtained again at weeks 3, 12, and 24.
The study authors provided this background. HIV associated neurocognitive impairment (HNCI), including subsyndromic neuropsychological impairment, minor cognitive motor disorder, and HAD (HIV-associated dementia), profoundly affects quality of life of people living with HIV. ARV therapy has reduced the incidence of HNCI, likely by decreasing HIV replication in the CNS. Despite this, the hprevalence of HNCI is rising, possibly because ARVs fail to completely suppress HIV replication in the brain. Ongoing replication may enable adaptation to neural cells, progressive brain injury, and ARV resistance. The pharmacokinetics, potency, and high barrier to resistance of LPV/r enable safe administration without other ARVs for limited durations, so similar to study 720 they administered LPV/r for 3 weeks to treatment-naïve individuals before adding additional ARVs.
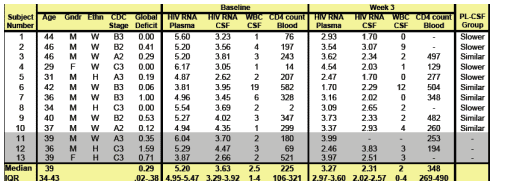
13 subjects enrolled in the study. Ten successfully completed the week 43 visit, the primary study endpoint, and were included in this analysis. Subjects were mostly middle-aged (median age 39), white (80%), men (90%) who had normal neuropsychological performance (80%). Six subjects had AIDS (CDC stage A3-c#) and 2 had impaired neuropsychological (NP) performance. Three subjects were excluded from analysis because of refusal of lumbar puncture, initiation of ARVs simultaneously, and poor adherence. Two subjects were withdrawn following week 3, one for hepatic ALT elevation and the other for virologic failure. Six subjects completed the week 24 visit & 2 remain actively enrolled.
At week 3, LPV/r reduced HIV RNA in CSF by a median 1.4 (IQR 1.0-1.6) and in plasma by a median 1.7 (IQR 1.6-2.4). After 3 weeks of LPV/r therapy alone, HIV RNA levels were below 1.7 in the CSF of 2 subjects and in the plasma in 1. Overall, HIV RNA declinded less in CSF than in plasma (median difference 0.5). Reductions of HIV RNA in the 2 fluids did not correlatew. In those who completed 12 weeks (n=7 to date), HIV RNA was below 1.7 in the CSF of all subjects and in the plasmaof 2. By week 24 (n=6 to date), HIV RNA was below 1.7 in both fluids of all subjects.
At baseline, the median CD4 count was 226 & the median HIV RNA levels were 3.63 in CSF & 5.20 in plasma. Phenotypic testing indicated lack of resistance to all study drugs. To further investigate the effectiveness of LPV/r in CSF, we compared the decline of HIV RNA in CSF & plasma. Between the baseline & week 3 visits, the differences between HIV RNA levels in CSF & plasma remained within 0.5 in 5 subjects (‘CSF similar’) but decreased decreased in the other 5, reflecting slower decline in HIV RNA in CSF than in plasma. At baseline, the ‘CSF similar’ group had higher CD4 counts (median 328 vs 76), higher HIV RNA levels in CSF (median 3.95 vs 3.23), lower HIV RNA levels in plasma (median 4.96 vs 5.54) and similar differences between HIV RNA levels in the two fluids (median 1.26 vs 2.26 , p.01).
Subject Characteristics at Baseline & week 3. 13 subjects enrolled but 3 11-13 were excluded from this analysis. Summary measures only include datafor the 10 subjects included in this analysis.
Baseline
HIV RNA in plasma: 5.20 log c/ml median
HIV RNA in CSF: 3.63 log c/ml median
Week 3
HIV RNA in plasma: 3.27 log c/ml median
HIV RNA in CSF: 2.31 log c/ml median
| |
| |
| |
 |
|
| |
| |
ABSTRACT
Background: Combination antiretroviral (ARV) therapy reduces HIV RNA levels in cerebrospinal fluid (CSF) and the incidence of HIV-associated dementia (HAD). Despite this, the prevalence of HAD is rising, possibly because ARV fail to completely suppress HIV in the brain. ARV may fail because an important component of many regimens, protease inhibitors (PI), extensively bind to plasma proteins, leaving little unbound drug to penetrate into the brain and CSF. However, these drugs are so potent that even low concentrations may inhibit HIV replication. The primary objective of this study was to determine whether a PI, kaletra (LPV/r), reduces HIV RNA levels in CSF when used by itself.
Methods: The study was an open-label, 24-week trial of sequential ARV therapy in which 16 ARV-naive subjects underwent screening, which included measurement of HIV RNA in CSF and plasma (RT-PCR) and HIV resistance in plasma (ViroLogic). Of the 16 subjects, 12 were enrolled and were started on LPV/r alone for 3 weeks. At least 2 other ARV were then added to complete 24 weeks. CSF was obtained again at weeks 3, 12, and 24. Data were analyzed using parametric and non-parametric tests, depending on data characteristics. All HIV RNA levels are in log10 copies/mL.
Results: At baseline, subjects had median CD4 counts of 188/ĶL and median HIV RNA levels of 3.5 in CSF and 5.4 in plasma. Phenotype testing indicated the lack of resistance to all study drugs. To date, 9 subjects have completed the week 3 visit, the primary study endpoint; 2 subjects withdrew (1 back pain, 1 non-adherence) and 1 is actively enrolled. At 3 weeks, LPV/r reduced HIV RNA levels in CSF (median 1.0, IQR 0.5 to 1.5, p = 0.008) and in plasma (median 1.6, IQR 1.4 to 2.1, p = 0.008). LPV/r reduced HIV RNA less in CSF than in plasma (paired t-test, p = 0.03) and reductions in CSF and plasma did not significantly correlate (r = 0.61, p = 0.14). In those who completed 12 weeks (n = 6 to date), HIV RNA was suppressed below detection in the CSF of 5 and in the plasma of 4. By week 24 (n = 3 to date), HIV RNA was suppressed below detection in both fluids of all subjects.
Conclusions: This study is novel because it used sequential introduction of ARV to determine whether a highly protein-bound PI can reduce HIV replication in the central nervous system. LPV/r alone reduced HIV RNA in CSF after just 3 weeks, proving that it contributes to control of HIV replication in the brain when used in combination with other ARV and suggesting that it may benefit patients diagnosed with or at risk for HAD.
|
|
| |
| |
| |
|
 |
 |
|
|