 |
 |
 |
| |
Liver Transplants in Hepatitis/HIV Coinfection at 12th CROI
|
| |
| |
Reported by Jules Levin
There were two studies presented, 1 in the USA (liver & kidney) & a 2nd in Germany (liver); both studies are reported in-depth below.
1- to 3-Year Outcomes in HIV-infected Liver and Kidney Transplant Recipients
Michelle Roland*1, D Stablein2, L Carlson1, L Frasseto1, B Murphy3, M Keller3, K Olthoff4, E Blumberg4, K Brayman5, R Redfield6, D Oldach6, B Barin2, and P Stock1
1Univ of California, San Francisco, USA; 2EMMES Corp, Rockville, MD, USA; 3Mt Sinai Sch of Med, New York, NY, USA; 4Univ of Pennsylvania, Philadelphia, USA; 5Univ of Virginia, Charlottesville, USA; and 6Univ of Maryland, College Park, USA
BACKGROUND
Liver and kidney failure are growing problems in the HAART era.
Solid organ transplantation may be safe & effective given:
--improvements in HIV-associated morbidity & mortality
--improvements in post-transplant immunosuppressive strategies & opportunistic infection prophylaxis
--hypothetical benefit of immunosuppressives in HIV-infected patients
Data from pre-HAART era show inconsistent but relatively poor outcomes.
Case series & small prospective studies in the HAART era suggest that patient & graft outcomes may be similar to those seen in HIV-uninfected patients.
We describe the largest cohort of HIV-infected transplant recipients in the HAART era with the longest followup.
METHODS
Prospective, multi-site cohort of HIV-infected transplant recipients followed for patient and graft survival, HIV outcomes (opportunistic complications, CD4+ T-cell count, HIV RNA) and rejection. Subjects had CD4+ T-cell count > 100/200 (liver/kidney) and undetectable HIV RNA or prediction of full suppression (liver). Time to subject and graft failure and cumulative rejection were analyzed using the Kaplan-Meier technique.
Opportunistic complication history excluded until protocol revision in April 2002 to allow most OIs with continued exclusion of PML, cryptosporidiosis & visceral KS. (one subject with a history of pulmonary KS is included).
DONORS: deceased & living donors were used.
Immunosuppression and rejection mgt: initial immunosuppression included either cyclosporine or tacrolimus with or without mycophenolate mofetil (MMF), in combination with steroids. Daclizumab use was allowed. Rejections were managed with a steroid pulse, changing calcineurin inhibitors or doses, and/or adding sirolimus and/or thymoglobulin.
All ARVs were allowed. AZT and d4T use was minimized due to in vitro data showing ART antagonism with MMF. Standard prophylaxis was employed for PCP, CMV, MAC, & candida.
RESULTS
29 subjects were enrolled between March 2000 & Sept 2003.
Age: 44-46
11 liver recipients & 18 kidney recipients, mostly men
55% white, Liver: 18% AA, 9% latino, 18% asian; kidney: 44% AA
1/11 liver recipients & 4/18 kidney recioients had prior OI complications.
Baseline CD$: 279 in liver group, 439 kidney.
HIV RNA: 9/11 undetectable in liver group, all undetectable in kidney group.
VIRAL HEPATITIS in LIVER GROUP
6/11 (55%) had HCV
5/11 had HBV (HbsAg+)
2/11 Hep B core Antibody positive.
In the kidney group 5/18 had HCV.
INDICATIONS FOR TRANSPLANT
Liver Recipients
HCV without HCC: 3 (27%)
HCV with HCC: 2 (18%)
HBV: 4 (36%)
HCV+HBV: 1 (9%)
DONOR CHARACTERISTICS
Living: 3 (27%)
Deceased: 6 (55%)
Deceased/high infectious risk: 1 (9%)
Deceased/other high risk: 1 (9%)
Kidney Transplants
HIVAN: 8 (44%)
Hypertension: 10 (56%)
Diabetes: 2 (11%)
Donor Characteristics
Living (related): 5 (28%)
Living (unrelated): 3 (17%)
Deceased: 6 (33%)
Deceased/high infectious risk: 4 (23%)
DEATHS-Survival Estimated (Kaplan-Meier)
There were 2 deaths (at 7 & 15 months) due to recurrent HCV among 11 liver transplant recipients followed for 701 (218-1364) days after transplantation.
There was 1 death (at 6 months) due to congestive heart failure among 18 kidney transplant recioients followed for a median of 869 (12-1664) days after transplantation.
The 1 & 3 year estimated patient survival rates for liver transplantation recipients were 91% & 81%, respectively.
The 1 and 3 year estimated patient survival rate for kidney transplant recipients was 94%.
Kaplan Meier Estimates of Graft Failure, without censoring death with function, from any cause among patients who received liver or kidney transplants
There was a graft loss among 11 liver transplant recipients (small for size graft lesion in living liver recipient) and 2 graft losses among 18 kidney transplant recipients (rupture due to severe acute rejection; chronic allograft nephropathy).
The 1 & 3 year graft survival rate for liver transplant recipients was 91% with censoring death with function and 82% without censoring death with function.
HIV DISEASE PROGRESSION
There was 1 case each of CMV (liver) and Candida (kidney) esophagitis and 1 anal carcinoma (liver); none occurred in subjects with a history of opportunistic infections. There was no significant change in CD4+ T-cell count in kidney (-27) or liver (+72) subjects. The last HIV RNA was undetectable in all but 1 surviving subject.
REJECTION
12 kidney subjects had 1 or more rejection; 1-, 2-, and 3-year cumulative kidney rejection estimates are 51%, 67%, and 78% (12%), and 1 (9%) liver recipient had rejection episodes.
The 1 & 3 yr cumulative rejection estimate for liver transplant is 10%. The 1, 2, & 3 yr cumulative estimates for kidney are 52%, 66%, and 77%.
Subjects who had 1 or more rejection episodes had a mean CD4 decrease of -111 while those without any rejection episodes had a mean increase in CD4s of 110.
KIDNEY FUNCTION
Median creatinine in kidney recipients=1.8 (IQR 1.3, 2.5).
Kidney recipients with rejection episodes have a higher mean creatinine than those without (2.8 vs 2.4).
LIVER FUNCTION
There has been no HBV progression among 5 recipients with HBV infection.
Among 6 recipients with HCV-infection, 4 have evidence of disease progression.
Median AST in liver recipients=36 (IQR 32, 194).
Liver recipients with HCV-coinfecvtion have a higher mean AST than those without (202 vs 32).
|
|
 |
| |
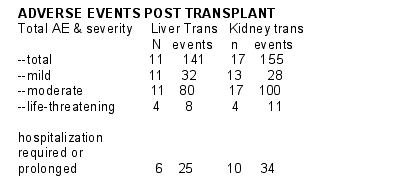
There were numerous changes in immunosuppression & ARV due to AE.
AUTHOR DISCUSSION
Overall graft & patient survival outcomes are promising even when compared to outcomes in the general transplant population; the trends continue to hold up over longer periods of follow-up than previously reported.
Areas of concern include potential high rates of HCV recurrence, also seen in HIV-negative transplant recipients, and high rates of rejection. Longer follow-up of more subjects will be required to determine if these trends are significant.
Additional areas in an ongoingf study enrolling patients at 19 US transplant centers include drug interactions, HPV-associated ano-rectal disease, HHV-8 associated disease. Clinical manifestations of other human herpes viruses, and outcomes in patients with 3TC resistant HBV or HIVAN.
At this stage of our research program, we believe it is reasonable to expect insurance reimbursement for liver & kidney transplants in people with HIV.
Orthotopic Liver Transplantation in HIV-positive Patients: Outcome of 10 Patients from the Bonn Cohort
Martin Vogel*, E Voigt, J C Wasmuth, H Brackmann, G Goldmann, M Wolff, A Hirner, T Sauerbruch, U Spengler, and J Rockstroh
Bonn Univ, Germany
HAART has improved the life expectancy of HIV-infected patients allowing orthotopic liver transplantation (OLTX) as a reasonable treatment option for selected patients with terminal liver disease.
We identified 10 patients who had been listed to Eurotransplant for liver-transplantation and known to be HIV-positive at the time of listing. A structured questionnaire comprising general demographic data, characteristics of HIV-infection and details of liver disease was used for assessing data corresponding to the time of listing for OLTX, the time of OLTX itself, (re)initiation of HAART after OLTX, and last follow-up.
Reasons for listing were acute fulminant hepatitis B infection (n = 2), and end-stage liver disease due to chronic hepatitis B virus (HBV) (n = 1), chronic HBV/hepatitis C virus (HCV) (n = 1), and chronic HCV infection (n = 6).
6 patients were hemophiliacs, 1 IVDU, 3 MSM. In the patients transplanted, CD4 counts ranged from 160 to 320. HIV RNA ranged from <50 to >500,000 copies/ml. MELD scores ranged from 15 to 25.
Patients with chronic liver disease waited a median of 315 days for transplantation and 2 patients died on the waiting list; in a third patient the liver function improved markedly after implementation of effective HAART so that the patient was removed from the waiting list.
Of the 7 patients who underwent transplantation, 1 patient died 84 days after OLTX due to intrathoracal hemorrhage while 6 patients are still alive at a median of 620 days. Steroid withdrawal was safely achieved in all patients at a median of 160 days post-OLTX with only 1 acute organ rejection observed in the early post-transplantation period.
Drug-to-drug interactions between protease inhibitors and cyclosporine A were observed and required substantial adjustments of cyclosporine A dosing to keep drug levels in the desired range.
The spectrum of postoperative complications did not differ from those of HIV-negative patients, however, KaposiÕs sarcoma in combination with multicentric Castleman disease took a complicated course in one patient.
Hepatitis C reinfection which occurred in all patients with preexisting hepatitis C was successfully treated by early interferon/ribavirin combination treatment. A fibrocholestatic course was observed in 2 patients. Presently, 1 patient shows sustained virologic response, 1 patient end of treatment and 2 patients early virologic response (>2 log reduction of HCV RNA by week 12).
CyA associated nephrotoxicity was observed in two patients who were subsequently treated with mycopheni=olate mofetil and reduced CyA doses in order to prevent further progression of renal insufficiency.
Of the 7 transplanted patients, 5 are currently on HAART, 1 patient was not on HAART, and a third patient paused therapy (all therapeutic measures were reduced to supportive fluid maintenance). HIV RNA is <50 c/ml in all patients on HAART & Cd4 counts range from 70 to 400, mostly in the 100-200 range.
The authors conclude, overall, our data strengthen the rationale for OLTX as a reasonable treatment option for selected HIV-positive patients. However, recurrent hepatitis C, opportunistic diseases and drug-to-drug interactions remain important issues to be resolved.
|
|
| |
| |
| |
|
 |
 |
|
|