 |
 |
 |
| |
Key clinical studies in initial and switch therapy
|
| |
| |
NATAP report CROI 2005
Dr Graeme Moyle MD, MBBS
Chelsea and Westminster Hospital, London
INITIAL THERAPY
-Initio Study
-ABCDE Study: prospective study comparing d4T vs abacavir for initial therapy, lipid/body changes- 96 weeks follow-up
-Meta Analysis of 64 studies for initial therapy comparing PI, NNRTI & triple nuc regimens
SWITCHING THERAPY
-NRTI sparing
-Switch to Tenofovir
-PI switches to atazanavir
Introduction
The 2005 Retrovirus Conference was not notable for new information with regards to initial therapy. Approaches to initial therapy are, however, changing as practice moves towards a preference to use thymidine sparing regimens upfront. Recent data, press released prior to the conference, reporting the 48 week results of the GS 934 study which compared Combivir (AZT/3TC) with Truvada (TDF/FTC), each in combination with efavirenz, have underlined the trend. Individuals who were randomised to initiate with the QD Truvada plus efavirenz regimen were significantly more likely to have an undetectable viral load at week 48 by intention to treat analysis than individuals have started on the BD Combivir based regimen. This outcome was largely driven by improved tolerability of the Truvada backbone. The data are similar to the outcome of CNA30024 which demonstrated that the backbone Epzicom (ABC/3TC, called Kivexa in the EU) also provided a similarly an effective and more predictably tolerable backbone than Combivir in initial therapy. Given the attractiveness of these new combinations pills which spare thymidine therapy, are dosed QD and do not appear associated with the development of lipoatrophy over prolonged follow-up is not surprising that there is also interested amongst both patients and physicians in switching people away from thymidine based regimens to thymidine sparing regimens.
Initial Therapy
INITIO
INITIO was 1 of 3 large strategy trials begun in 1998/1999 comparing HIV treatment starting with a 3-drug regimen containing a non-nucleoside reverse transcriptase inhibitor (NNRTI) followed by a regimen with a protease inhibitor (PI) for failure or a PI (followed by a regimen with a NNRTI for failure) and a 4-drug regimen containing both NNRTI plus PI. The other studies, ACTG 384 and FIRST have already reported their outcomes. The problem with all of these trials has been to include backbones of d4T plus ddI. As was very clearly shown in ACTG 384, this backbone has very poor tolerability not only with regards to the development of clinical lipoatrophy but also a range of other toxicities often secondary to mitochondrial dysfunction. These include peripheral neuropathy and pancreatitis. The resulting large numbers of discontinuations due to toxicity complicate the interpretation of these studies and the individualisation into 2005 therapy. They also underline the speed of evolution of antiretroviral therapy.
The initio study randomised individuals to receive either didanosine/stavudine/efavirenz (ddI/d4T/EFV) followed by zidovudine/lamivudine/abacavir/nelfinavir (ZDV/3TC/ABC/NFV)(arm 1) was compared to ddI/d4T/NFV followed by ZDV/3TC/ABC/EFV(arm 2) and a four drugs three classes regimen of ddI/d4T/EFV/NFV with no specified second regimen (arm 3) in treatment-naive patients. The primary outcome measures were HIV RNA < 50 copies/mL and the change from baseline in CD4 count at 3 years (analysis censored for missing values). Secondary outcome measures included change from baseline in HIV RNA at 3 years, progression to new AIDS events or death and incidence of adverse events. Time on initial regimens allowed for within-class drug substitutions for drug intolerance. The study randomized 915 patients (300 arm 1, 311 arm 2, 304 arm 3) and followed them for a median of 192 weeks when the trial closed in June 2004. At baseline, mean age was 39 years, 21% had previously experienced an AIDS-defining event, median CD4 count was 200 cells/mm3 (interquartile range (IQR) 80 to 329) and mean HIV RNA 4.93 log10 copies/mL. The proportion of time on the initial regimen (including substitutions) was 74%, 63%, and 51% in arms 1, 2 and 3, respectively. By intent-to-treat analyses at 3 years the proportion of patients with HIV RNA < 50 copies/mL was 74%, 62%, and 62% (global p = 0.004). The median change in CD4 count was +271, +275, and + 258 (p = 0.7). There were no significant differences between groups in progression to a new AIDS event/death, in the number of patients with at least one serious adverse event or with at least one adverse event stopping one or more HIV drugs. At the end of follow-up 18 percent of individuals randomised to arm 1, 41 percent of individuals in arm 2 and (of course) 100 percent of individuals and arm 3 had been exposed to all three oral drug classes. Changes to the nucleoside backbone were made by 135 individuals in arm 1, 99 individuals in arm 2 and 117 individuals in arm 3, with the most common modification being to replace d4T/ddI with AZT/3TC
In keeping with the ACTG 384 study (and also be smaller CLASS study), Initio provides evidence of the superiority of efavirenz based regimens for initial therapy over unboosted protease inhibitor regimens and over three class for drug regimens. In keeping with multiple prior studies there appears to be no advantage to using a fourth drug in initial therapy and in many cases the addition of a fourth drug leads to higher rates of adverse event related discontinuation and may create particular challenges with regards to establishing a second line of therapy. The large number of adverse event in the study that were related to the d4T/ddI backbone underline the importance of avoiding this combination in clinical practice.
ABCDE
The ABCDE study is a randomised comparative study of abacavir (ABC) and stavudine (d4T), both combined with lamivudine (3TC)/efavirenz (EFV), as initial therapy. The study is of considerable interest due to the long-term follow-up to 96 weeks, the inclusion of prospectively collected data with regards to the development of lipoatrophy (including by serial DEXA scan), and the similarity of the design of the study to the Gilead 903 study. ABCDE included 237 treatment naive individuals in a randomized, open label manner . The baseline characteristics included a mean CD4 was 213/ÁL and mean HIV-1 RNA 5.2 log. Over 96 weeks of follow-up ABC was superior in virologic response by ITT (60.9% vs 47.5%, p = 0.05) but not by as treated analysis (87.5% vs. 85.3%, p = 0.81). this is in contrast to the GS 903 study in which similar virological efficacy was seen by both intention to treat and as treated analysis for tenofovir vs d4T. The mean CD4 count increase was similar, with a gain of more than 250 cells/ÁL in both arms. Over 96 weeks of follow-up both groups gained weight (a mean weight increase of 4.7 and 2.5 kg for ABC and d4T arms, respectively (p = 0.06)), however DEXA scan data performed at baseline, 48 and 96 weeks in 59 patients, showed that patients randomised to d4T lost 1579 g of limbs fat over time whereas individuals randomised to abacavir gained 913 g of limb fat (p < 0.001). These findings are in keeping with the recently published lipodystrophy results from the CPCRA FIRST study. With regards to metabolic outcomes, patients randomised to d4T experienced a greater increase in triglyceride levels (p = 0.03), a smaller increase in HDL cholesterol (p < 0.001) and in ApoA1 (p < 0.001), and a less favourable change in TC:HDLc ratio (p = 0.005) as compared with abacavir recipients. The abacavir group demonstrated good long-term tolerability with only 20 (17.4%) of the originally randomised patients discontinuing due to adverse events, with 13/20 of these events being secondary to an initial hypersensitivity reaction. In contrast, 33(27%) of d4T recipients discontinued due to adverse events, the most common (14/33) reason that means secondary to clinical lipoatrophy. The differences in this continuation rate were statistically significant. This study epitomises the attractiveness of thymidine sparing regimens as initial therapy and underlines the attractiveness of the abacavir plus 3TC backbone.
Meta-analysis of initial therapy studies
With so many options available for initial therapy it is impossible to compare all of the options in appropriately powered randomised controlled studies. Another way of assessing what might be the best approach to therapy is to combine data from multiple studies into a meta-analysis. Results of an analysis of 64 clinical trials involving 10,559 subjects over 85 different treatment arms was reported. The study included trials which have evaluated triple drug combinations. Triple drug combinations included 2 nucleoside reverse transcriptase inhibitors (NRTI) plus a protease inhibitor, boosted PI, non-nucleoside RTI (NNRTI), or a third NRTI. The primary endpoint for the analysis was the proportion of patients with an HIV-1 RNA less than 50 copies/ml at week 48 by intention to treat analysis. The study found that significantly greater percentages of individuals achieved optimal virological response when using a regimen combining 2 NRTIs combined with either a boosted protease inhibitor (64 percent) or an NNRTI (63 percent) relative to an unboosted protease inhibitor (44 percent) or a third nucleoside analog (51 percent). Changes in CD for cell count favoured the boosted protease inhibitor group (+209 cells) as compared to be NNRTI group (+180), unboosted protease inhibitor (+178) and triple nucleoside analog group (+150). In contrast to a previous meta analysis of initial therapy trials pill burden was not noted to be a significant predictor of virological response.
Limited data are currently available directly comparing initial therapy with an NNRTI relative to a boosted protease inhibitor. Such studies are ongoing, however, this meta-analysis suggests that either approach is reasonable from an efficacy standpoint for initial therapy.
Switch Studies
NRTI-sparing
Studies evaluating switching between regimens in individuals currently receiving successful antiretroviral therapy followed two main themes. Firstly studies switching from a thymidine analog to a alternative nucleoside backbone containing either abacavir or tenofovir, and secondly, switching away from a nucleoside analog based regimen to one based on a boosted protease inhibitor and a NNRTI. In general, these switches were virologically and immunologically successful so that the focus of much of the outcome was with regards to the resolution or prevention of toxicities, most notably lipoatrophy.
In ACTG study A5116 individuals who have been previously treated with two nucleoside analog plus a protease inhibitor (indinavir or nelfinavir, in some cases with efavirenz also included in the regimen) were randomized to modify their therapy by either replacing the protease inhibitor with the efavirenz or to modify the combination to a nucleoside analog sparing regimen consisting of lopinavir/r with efavirenz. The lopinavir/r doing was adjusted to 4pills BD to compensate for the known drug interaction with efavirenz. Patients entering the study had been on their prior treatment regimens for at least 18 months and had undetectable viral loads. Patients were not required to have clinical lipoatrophy to enter this study although the limb fat mass, based on a DEXA scanned some study, was moderately reduced at 6 kg (normal ~8 kg). The study included 236 individuals evenly randomized with a follow-up of 110 weeks. The majority of individuals (78%) in the two nucleoside analog plus efavirenz armed were receiving AZT/3 TC as their nucleoside backbone. Discontinuationof randomized to therapy was significantly more common in individuals given lopinavir/r plus efavirenz. Time to treatment failure (a composite endpoint of virological failure and toxicity related treatment discontinuation) was significantly delayed in individuals randomized to receive to nucleoside analogues plus efavirenz relative to the nucleoside sparing combination (p < 0.001). A trend to more virological failures was noted in the nucleoside sparing arm. Nucleoside analog sparing regimen, however, was associated with gradual that recovery overtime whereas individuals who continued on a thymidine based regimen lost fat overtime. Importantly, the rate of fat loss appeared similar in individuals who continued on a regimen containing AZT to those who received a combination based on d4T as their thymidine analog choice. These data underline the emerging recognition of the role played by AZT in new incidents of clinical lipoatrophy.
A second A CTG study, 5110s, evaluating the switch from a thymidine analog to abacavir (the current standard of care for managing lipoatrophy) with switching to a nucleoside sparing regiment consisting of dose adjusted lopinavir/r plus nevirapine. The study included 101 individuals with clinical lipoatrophy, with 77 patients switching therapy and a further 24 remaining on their thymidine based regimen as a control arm. Individuals are required to have clinical lipoatrophy and a currently undetectable viral load at entry. The majority of individuals (76%) were receiving d4T at study entry. Both switches were associated with improvements in subcutaneous abdominal fat although thigh fat, as measured by a CT scan, increased more substantially in individuals receiving the nucleoside analog sparing regimen. Maintenance of virological control was similar across all groups.
One key downside of both of these studies evaluating nucleoside analog sparing regimens was that despite improvements in peripheral fat mass there was a marked worsening in the lipid profile, in particular elevation is in triglycerides and to a lesser extent total cholesterol. Switching to abacavir, in general, is not associated with a substantial worsening of the lipid profile. This key difference is likely to influence decisions with regard to treatment switching when the options of just switching to a thymidine sparing nucleoside analog containing regimen vs switching to a nucleoside sparing regimen are considered any clinical practice.
Switching to Tenofovir
Several studies reported data evaluating the switch from a thymidine analog to tenofovir. The data were remarkably consistent. Patients switching were observed to have increases in limb fat, reductions in lactate and improvements in the overall lipid profile. Additionally, recovery in haemoglobin was noted in individuals who switched away from AZT to tenofovir and improvements in mitochondrial function or PBMC mitochondrial DNA content were observed.
Two Spanish studies reported data regarding the switch from d4T to tenofovir. In the larger these two studies, 58 patients were randomized to either continue on d4T(n=20), reduce their dose of d4T to 30 mg BD (n=18) or switch to tenofovir (n=18). At 6 months, all patients but 1 (d4T40 group) had a plasma HIV RNA < 200 copies/mL. Significant changes in laboratory parameters, were observed with regards to triglycerides (mean, mg/dL) (+19, d4T40; -40, d4T30; -133, TDF; p = 0.02) and total cholesterol (mean, mg/dL) (+4, d4T40; -4, d4T30; -28, TDF; p = 0.04). Mean changes in total (-597, d4T40; +332, d4T30; +1005, TDF; p = 0.04) and limb fat in grams (-247, d4T40; +77, d4T30; +440, TDF; p = 0.008) for a significantly differed among groups. The study suggeststhat the individuals intend the option as switching of away from d4Tis not an option than it may be feasible to reduce d4T dose. However, the observations indicate that switching from d4T to tenofovir is much more effective from both a limb fat and lipid standpoint.
The findings were reiterated in a 53 patient cohort study in which patients switched from d4T to tenofovir. The specific interest in the study was not only did limb fat mass and lipid profile improved but also PBMC mitochondrial DNA content was noted to increase by approximately 50% over an 18 month period and that using facial ultrasound, individuals were noted to have an increase in the volume of their malar (Bichat) fat pad. This is the first study to have reported that facial fat mass increases after treatment switching. Details of the key outcome parameters in study are shown in the table below.
|
|
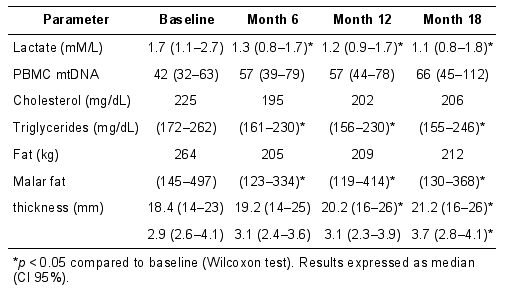 |
| |
The key question that follows these data is whether there are differences between tenofovir and, the established standard of care for treatment switching, abacavir with regards to metabolic and morphological parameters when these agents are used as replacements for a thymidine analog. As both abacavir and tenofovir are available in fixed dose formulations for once daily administration, consideration of switching to these agents from a twice daily thymidine analog may provide benefits to individuals not only with regard to the prevention or recovery from adverse events that may also provide adherence support through facilitating at the low pill burden once daily regimen.
A randomized, open-label, 48-week study assessed change in limb fat following substitution of AZT or d4T with ABC (300mg BD) vs. TDF (300mg QD) in adults on HAART with moderate/severe lipoatrophy who are naive to ABC and TDF with a current viral load <50c/mL. Limb fat was measured by dual-energy x-ray absorptiometry (DEXA); secondary end points included HIV RNA, adverse events, visceral fat mass (by CT scan), and fasting metabolic parameters. Analyses were performed on an intent-to-treat basis ignoring treatment changes.
The study, known as RAVE, included 105 adults receiving D4T (n=71) or AZT (n=34) were randomized, 53 to ABC, 52 to TDF. Limb fat mass was similar at baseline (median : 3.29 kg in ABC and 3.0kg in TDF). At week 48 there was a significant increase in limb fat in both groups from baseline values (p<0.01) but no difference between drug arms (increases of 0316g vs. 393g; p=0.97, difference in means: 0.2, 95% confidence interval: -0.2, 0.5). Of note, individuals switching from both d4T and from AZT were observed to recover fat. Similar changes in visceral fat and subcutaneous abdominal fat by CT were observed. Virological suppression was similarly maintained between both groups. Changes in total cholesterol, LDL and triglycerides through week 48 significantly favoured TDF. No significant differences between treatment groups were observed in changes in renal function or bone mineral density scores by DEXA. Patients who switch from AZT were noted to have substantial increases in haemoglobin, by 0.45g.dl when switched to ABC and by 0.85g/dl when switch to TDF.
The data establish switching to TDF as an alternative standard of care for the management of lipoatrophy HIV-infected adults. Both switches lead to similar, significant increases in limb fat over 48 weeks. While both agents maintain virological suppression, TDF is associated with fewer treatment discontinuations and greater improvements in lipid parameters than ABC.
PI switches
Specifically regarding the management of lipid elevation, previous studies have demonstrated that modification treatment from a protease inhibitor based regimen to one combining to nucleoside analog as with either efavirenz, nevirapine or abacavir may lead to improvements in lipid profile. However, for individuals who have previously failed a nucleoside analog or NNRTIs containing regimen this switching approach is not an option. Atazanavir is a protease inhibitor with a favourable lipid profile established from both studies in treatment naive and treatment experienced settings. A 48 week open-label randomized study evaluated the switching of individuals from a PI based regimen(either boosted (~40%) or unboosted (~60%)) to atazanavir (ATV) 400 mg QD (n=126) for continuation of the PI based regimen (n=118). Patients entering study were required to have had elevated LDL cholesterol greater than 130 mg/dl. Switching to atazanavir was associated with significant decreases in total and LDL cholesterol and in triglycerides (15%, 18%, and 35% reductions for each of these parameters, respectively). Virologic suppression was maintained, with 2 (ATV) and 1 (PI) viral load rebounds through week 12. rates of adverse events did not differ between the groups.
The magnitude of these reductions in similar to that observed following the introduction of lipid lowering therapy. As a result a substantial proportion of patients in the atazanavir group achieved NCEP targets without the need for further consideration of intervention with lipid lowering therapy.
Conclusions
Studies presented at the 2005 retrovirus conference suggest that thymidine sparing regimens may be preferred as initial therapy and may be attractive as switch options for individuals currently receiving a thymidine based Regimen. New combination tablets involving abacavir and lamivudine, tenofovir and emtricitabine provide compact QD backbones which are associated with favourable metabolic and morphological outcomes, fewer haematological toxicities, greater CD4 recovery and at least a similar virological efficacy to thymidine based regimens. These large number of advantages suggest that these combinations should represent the standard of care backbones for antiretroviral therapy. Patients currently receiving thymidine based regimens should consider treatment modification to gain advantage from these new formulations.
For individuals experiencing dyslipidaemia on HIV protease inhibitor, consideration should be given to simplification of their backbone to one containing atazanavir.
|
|
| |
| |
|
 |
 |
|
|