 |
 |
 |
| |
Heart Disease and Mortality Trends in People With HIV
|
| |
| |
A Report from the 10th European AIDS Conference (EACS),
November 17-20, 2005,
Dublin
Mark Mascolini
With so many ominous links between antiretrovirals and cardiovascular disease making recent headlines, one can be excused for assuming that researchers have closed the book on ties between therapy and a threatened heart. But nothing could be further from the truth, according to results of two randomized antiretroviral trials unveiled at EACS.
Meanwhile, mortality studies in two vast HIV cohorts confirmed some suspicions and rendered more than one surprise.
Antiretrovirals improve arterial function
Work reported 4 years ago linked protease inhibitors (PIs) to dangerously impaired endothelial function [1]. In this study James Stein used ultrasound to gauge flow-mediated vasodilation of the brachial artery-a standard technique to estimate arterial elasticity-in 37 people taking antiretrovirals. The 15 people using a non-PI regimen had relatively good flow-mediated vasodilation (8.1 + 6.7%), while the 22 PI-treated people had stiff arteries signaled by a vasodilation score of 2.6 + 4.6% (P = 0.005). But the study was small and checked people at a single time point, so Stein couldn't tell how good-or bad-artery walls looked before these people started antiretrovirals or as treatment continued.
An AIDS Clinical Trials Group (ACTG) team will try to plug this research gap in an ongoing study of 82 antiretroviral-naive people randomized to one of three regimens-efavirenz plus two nucleoside reverse transcriptase inhibitors (NRTIs), lopinavir/ritonavir plus two NRTIs, or lopinavir/ritonavir plus efavirenz [2]. Francesca Torriani from the University of California, San Diego reported that the study group's median age stood at 35 years (young for an HIV cohort in the US), their median CD4 count measured 244 cells/mL, and their median viral load was 4.82 log copies/mL.
Torriani and colleagues at six ACTG sites excluded people with a history of cerebrovascular disease or diabetes and anyone taking antilipid drugs, antioxidants, or steroids. Like Stein, they used high-resolution ultrasonography to grade flow-mediated vasodilation of the brachial artery-an artery in the upper arm. Unlike Stein, they found that everyone had dangerously low flow-mediated vasodilation before they even started antiretrovirals (Table; normal is 11%). Yet that metric improved significantly after only 4 weeks of therapy, and the improvement continued through 24 weeks of follow-up.
Average flow-mediated vasodilation in people starting antiretrovirals
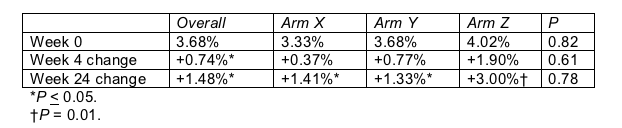
Antiretroviral assignment had not been disclosed at week 24, but Torriani reported that vasodilation improved significantly with all three regimens tested, designated Arms X, Y, and Z. Although she offered no opinion on between-arm vasodilation differences, it appears that people in Arm Z are improving most. Lipid improvements did differ significantly from arm to arm, but Torriani didn't say whether Arm Z surpassed the others by lipid measures.
The ACTG 5152S team suggested that improved endothelial function in this study may signify a lower short-term risk of cardiovascular disease.
Concerns that years of antiretroviral therapy will kill more and more people with heart attacks rest mainly on results from the multicohort D:A:D study, which reckoned a 17% jump in myocardial infarction risk for every extra year of treatment [3]. It's easy to forget that a similarly massive cohort study, this one involving the US Veterans Administration (VA), charted dwindling hospital admission rates for cardiovascular or cerebrovascular disease among people taking antiretrovirals from 1995 to 2001 [4]. In a review lecture at EACS, Düsseldorf HIV clinician Stefan Mauss noted that an additional 3 years of follow-up in the VA cohort still failed to turn up any hint that prolonged antiretroviral therapy poses a serious threat of heart disease. The VA update will probably get aired at a big HIV meeting soon.
Replacing a PI with atazanavir:
Separating swans from ducklings?
The SWAN study, a 48-week open-label trial that randomized people to continue an effective PI regimen or switch to atazanavir, found fewer viral rebounds and better lipid trends in the atazanavir group [5]. But this SWAN paddled primarily through the backwaters of antiretroviral history because nearly half the study participants entered the trial taking an outmoded unboosted PI, and almost everyone who switched to atazanavir took it without ritonavir.
Big trials take a long time to mount, run, and analyze, so no one can blame the investigators for presenting anachronistic results. But with ritonavir-boosted atazanavir the standard of care, SWAN ends up giving clinicians lots of data they can't use.
Jose Gatell from Barcelona's University Hospital Clinic and colleagues across Europe recruited 419 people taking their first or second PI regimen. Almost everyone had a viral load under 50 copies/mL, and no one had a PI failure on their charts. Baseline CD4 counts averaged 490 cells/mL, and 30% had HCV coinfection.
PI experience averaged 3.4 years, and 193 people (46%) were taking an unboosted PI, usually nelfinavir (72% of the unboosted groups), but also indinavir (18%) and even saquinavir (6%). Among the 226 people who signed up for SWAN while taking a boosted PI, 153 (68%) were taking lopinavir/ritonavir.
SWAN investigators randomized these people in a 2-to-1 ratio to trade their PI for atazanavir or to keep their current PI. Of the 278 people randomized to atazanavir, only the 10% taking tenofovir used 300/100 mg of atazanavir/ritonavir once daily. The rest took 400 mg of atazanavir without ritonavir.
After 48 weeks an intent-to-treat analysis of people who started their assigned regimen found significantly fewer confirmed rebounds above 50 copies/mL in the atazanavir group (Table). The 95% confidence interval in this overall analysis allowed statisticians to say that atazanavir is virologically noninferior to the baseline PIs, not that it is virologically superior.
Switching to atazanavir from a boosted or unboosted PI
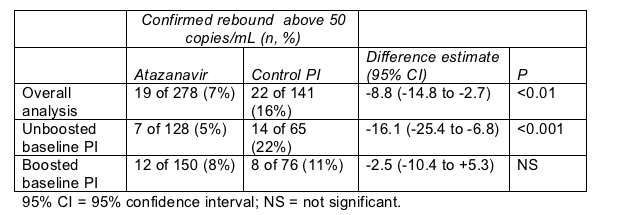
Perhaps not surprisingly, the lower rebound rate after the tradeoff to atazanavir can be traced almost entirely to people who started SWAN taking a passe unboosted PI (Table). Among people who entered the trial taking a ritonavir-propped PI, switching to atazanavir did not result in tighter viral control (Table).
The same proportion in each treatment arm, 6%, quit because of side effects or other "adverse events." Scleral icterus and jaundice affected 29 people (11%) taking atazanavir and none who stuck with their baseline PI. Diarrhea troubled 8 people (6%) taking a control PI and 5 (2%) switching to atazanavir (P < 0.05).
In the atazanavir group 43% had grade 3 or 4 leaps in total bilirubin, compared with 3% in the control group. Among people with HCV coinfection, 53% who switched to atazanavir had grade 3 or 4 total bilirubin buildups, compared with 8% in the control arm. Liver enzyme jumps proved similar in the two study arms after 48 weeks, regardless of HCV coinfection.
Equivalent proportions in the two treatment groups had total cholesterol at or above 240 mg/dL and triglycerides at or above 250 mg/dL when the trial began. After 48 weeks significantly fewer people switching to atazanavir had cholesterol or triglyceride readings that high (8% versus 29% for total cholesterol, P < 0.0001; 9% versus 30% for triglycerides, P < 0.0001).
As with the virologic outcomes, these side effect results do not readily translate into current practice because atazanavir levels soar so much higher with a ritonavir kick. Much work remains in defining the toxicity profile of boosted atazanavir. A Bristol-Myers Squibb study reported at the Lipodystrophy Workshop just before EACS, for example, found equivocal evidence on boosted atazanavir and glucose regulation [6].
Bristol researchers had 24 healthy volunteers take standard doses of atazanavir/ritonavir or lopinavir/ritonavir for 10 days. After a week or more taking no PIs, the volunteers switched to the alternate regimen. Glucose area under the curve rose significantly with lopinavir/ritonavir but not atazanavir/ritonavir. But insulin climbed significantly with both regimens, and mean glucose response during an oral glucose tolerance test rose almost identically with atazanavir/ritonavir (111.1 mg/dL) and lopinavir/ritonavir (110.6 mg/dL) (P < 0.05 versus baseline for both).
Mulling these results at EACS, Andrew Carr from Sydney's St. Vincent's Hospital opined that atazanavir/ritonavir probably does cause insulin resistance. Whether one can blame these glucose upsets solely on the ritonavir boost hardly matters if that's the way atazanavir gets prescribed.
Notably, this study in healthy volunteers without HIV infection also charted significant drops in "good" high-density lipoprotein cholesterol with 10 days of either lopinavir/ritonavir or atazanavir/ritonavir (P < 0.01), a blot on atazanavir's heretofore unsmirched lipid record.
Why people with HIV die (or don't) today
Two big cohort studies yielded two surprises about mortality among people taking potent antiretrovirals for 5 years or more:
- CASCADE cohort researchers reported that-contrary to results of other studies-opportunistic infections remain the leading cause of death among people with HIV [7].
- APROCO and Aquitaine cohort collaborators found that people who attain and maintain a CD4 count above 500 cells/mL live as long as people without HIV [8].
Soaking up data from 22 cohorts of people with known HIV seroconversion dates, the CASCADE crew set out to compare causes of death in the pre-HAART era with causes since potent regimens gained wide use in developed countries. Of the 7680 cohort members included, 1962 died, 504 of them from unknown causes.
Ronald Geskus from the Health Service of Amsterdam reported that rates of progression to almost all causes of death dropped-as one would expect-since strong triple therapies arrived. After about 15 years of follow-up in both the pre-HAART and the HAART groups, opportunistic infections proved the most common killer in both eras. But cumulative incidence of death from opportunistic infection during the days of HAART was only about 25% the incidence in pre-HAART years.
After opportunistic infection the most frequent relative causes of death changed greatly when muscular regimens gained sway (Table):
Leading causes of death in the CASCADE cohort
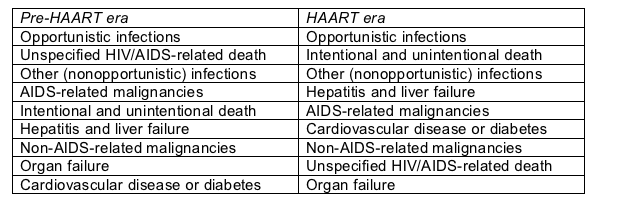
The biggest shifts in the HAART era involved a hefty relative jump in intentional and unintentional death (which are probably nice ways to say suicide and murder), a higher relative rate of death from liver failure, and a higher relative rate of death from heart disease or diabetes.
Geskus and colleagues reported that the greater relative frequency of intentional and unintentional death in the HAART epoch reflects a significant jump in such deaths among injecting drug users. They call the relative surge in liver-related deaths a nonsignificant trend.
Together the APROCO and Aquitaine cohorts embrace 1743 men and 536 women who began a PI regimen from 1997 through 1999 and logged 5 years of follow-up. Charlotte Lewden from INSERM Unit 593 in Bordeaux reported that the combined group had a median 4.4-year duration of HIV infection before treatment, a median pretreatment CD4 count of 270 cells/mL, and a median pretreatment viral load of 4.5 log copies/mL. Lewden and colleagues documented HCV coinfection in 27% of these people, and 21% had AIDS when they started their PI.
Death rates per 100 person-years skidded from 11.3 in 1996 to 5.1 in 1997, 3.5 in 1998, 1.8 in 1999, and 1.9 in 2000. After that, though, mortality inched back up to 3.8 per 100 person-years in 2003. Lewden did not parse this uptick.
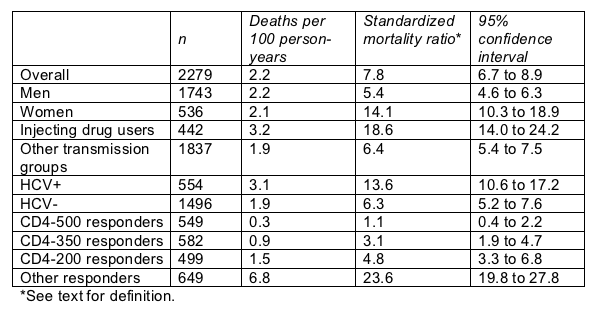
The French team figured a standardized mortality ratio as observed deaths divided by expected deaths-stratified by age and gender-with 1999 French death rates as the point of reference. The overall standardized mortality ratio showed a 7.8 times higher death risk in the HIV cohorts than in the general population and even higher risks for injecting drug users (18.6 times), women (14.1 times), and people with HCV coinfection (13.6 times) (Table).
But people with at least two recorded CD4 counts above 500 cells/mL, no recorded count under 500 cells/mL, and no viral load at or above 100,000 copies/mL had a standardized mortality ratio of 1.1 compared with the general population. In other words, the difference between their death rate and that of the general population-if there is a difference-must be negligible.
Mortality rate in 2279 French cohort members: 1/1997 to 6/2003
Lewden and coworkers argued that their findings mean HIV infection "might no longer be considered as an obstacle to obtain insurance contracts and loans" if a person reaches and keeps a CD4 count above 500 cells/mL.
References
1. Stein JH, Klein MA, Bellehumeur JL, et al. Use of human immunodeficiency virus-1 protease inhibitors is associated with atherogenic lipoprotein changes and endothelial dysfunction. Circulation 2001;104:257-262.
2. Torriani FJ, Parker RA, Murphy FL, et al. Antiretroviral therapy improves endothelial function in treatment-naive HIV-infected subjects: a prospective, randomized multicenter trial (A5152S). 10th European AIDS Conference. November 17-20, 2005. Dublin. Abstract PS5/3.
3. El-Sadr W, Reiss P, De Wit S, et al. Relationship between prolonged exposure to combination ART and myocardial infarction: effect of sex, age, and lipid changes. 12th Conference on Retroviruses and Opportunistic Infections. February 22-25, 2005. Boston. Abstract 42.
4. Bozzette SA, Ake CF, Tam HK, et al. Cardiovascular and cerebrovascular events in patients treated for human immunodeficiency virus infection. N Engl J Med 2003;348:702-710.
5. Gatell J, Salmon-Ceron D, Lazzarin A, et al. Efficacy and safety of atazanavir based HAART in patients switched from a stable boosted/unboosted protease-inhibitor treatment: the SWAN Study. 10th European AIDS Conference. November 17-20, 2005. Dublin. Abstract PS1/1.
6. Noor MA, Flint OP, Parker RA, et al. Evaluation of insulin sensitivity in healthy volunteers treated with low-dose ritonavir combined with atazanavir or lopinavir: a prospective, randomized study using hyperinsulinemic, euglycemic clamp and oral glucose tolerance testing. 7th International Workshop on Adverse Drug Reactions and Lipodystrophy in HIV. November 13-16, 2005. Dublin. Abstract 16.
7. Geskus R, Geskus R, Porter K, et al. Has effective therapy altered the spectrum of cause-specific mortality following HIV seroconversion? 10th European AIDS Conference. November 17-20, 2005. Dublin. Abstract PE18.4/6.
8. Lewden C, APROCO-COPILOTE Study Group. Responses to antiretroviral treatment over 500 CD4/mm3 reach same mortality rates as general population: APROCO and Acquitaine cohorts, France. 10th European AIDS Conference. November 17-20, 2005. Dublin. Abstract PE18.4/8.
|
|
| |
| |
|
 |
 |
|
|