 |
 |
 |
| |
Lipoatrophy Improved: RAVE Study
|
| |
| |
Reported by Jules Levin
10th European AIDS Conference (EACS)
November 17-20, 2005
Dublin, Ireland
Poster Number PE 9.3/2
"Factors Associated with Limb Fat Recovery in a Prospective Randomised Comparative Study of Thymidine Replacement with either Tenofovir DF or Abacavir in Persons with Clinical Lipoatrophy The RAVE Study'
G Moyle1 , C Sabin2, J Cartledge3, M Johnson2, E Wilkins4, D Churchill5 , P Hay6, A Fakoya7, M Murphy8, G Scullard9, C Leen10, and G Reilly11 for the RAVE study
group UK
1 Chelsea and Westminster Hosp, London; 2 Royal Free & UC Medical School,
London; 3 UCL London, 4 North Manchester Hosp; 5 Lawson Unit Royal Sussex
County Hosp; 6 St Georges Hosp London; 7 Newham General London; 8 St
Barts & The London; 9 St Mary's Hosp London; 10 Western General Hosp Edinburgh; and 11 Gilead Sciences Inc.; UK
Thymidine analogue therapy is associated with peripheral fat loss and dyslipidemia
- Removal of Thymidine analogues leads to gradual fat recovery although the rate of fat recovery varies between individuals
- Host, HIV and treatment factors that may infl uence fat recovery have not been identified
Figure 1. Design
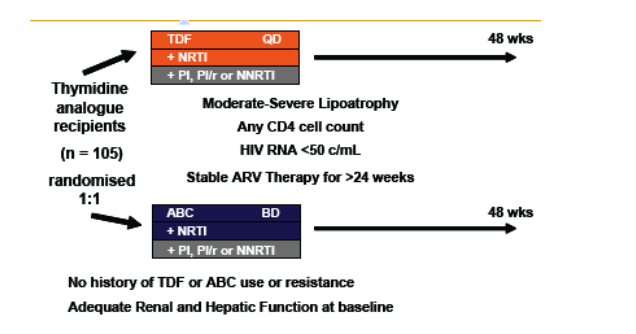
Statistical Methods:
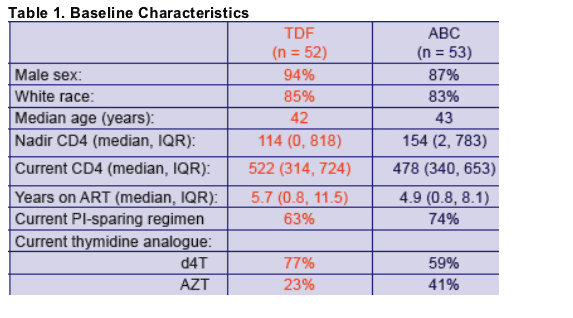
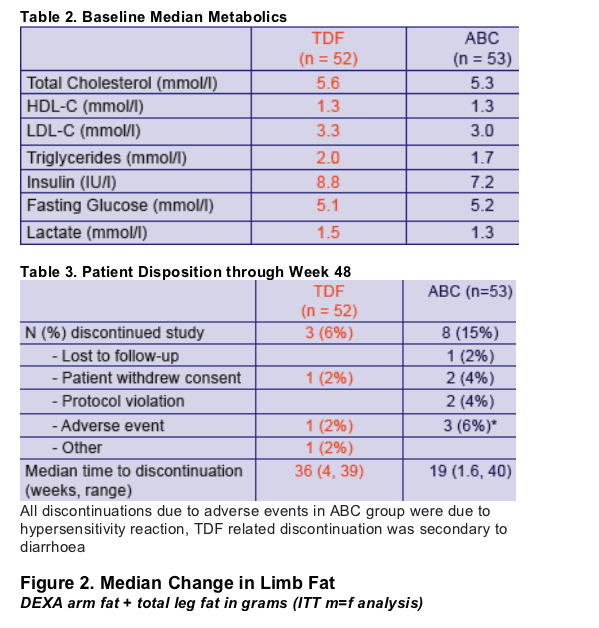
- Trial endpoints: change in total limb fat mass (by DEXA) and lipids from baseline to 48 weeks
- Changes in the 2 treatment groups were compared using unpaired t-tests
- Factors associated with absolute change in limb fat (Model 1) and with a recovery of >710g limb fat (model 2)over 48 weeks were identified using multiple logistic regression
- Factors considered were randomization group, age, sex, risk group, ethnicity, baseline CD4, nadir CD4, CDC status, baseline limb fat, baseline thymidine analog, the number of antiretrovirals previously received, time on therapy, weight at randomization and use of PI or NNRTI as the third agent in the regimen.
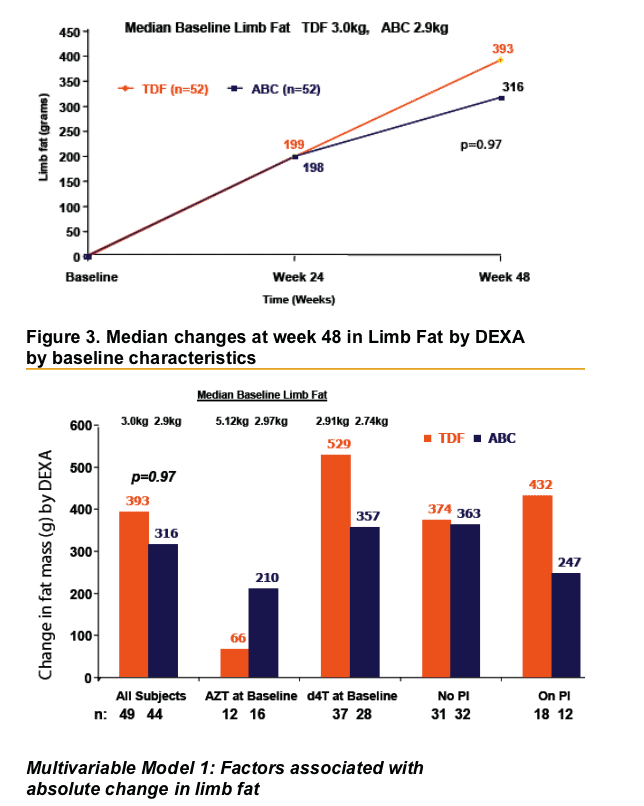
- Age at randomisation Per 5 years older: 103g less fat recovery by Week 48 (95%CI 214g less to 8g more p=0.07)
- Baseline Thymidine analogue ZDV at baseline: 419g less fat recovery at Week 48 (95%CI 65g-774g less, p=0.02)
Factors considered: randomization group, age, sex, risk group, ethnicity, baseline CD4, nadir CD4, CDC status, baseline limb fat, baseline thymidine analogue, the number of antiretrovirals previously received, time on therapy, weight at randomization and use of PI or NNRTI as the third agent in the regimen.
Multivariable Model 2: Odds ratios for factors
associated with >710g limb fat recovery over 48
weeks
- Baseline Thymidine analogue ZDV at baseline OR 0.15 (95%CI 0.03-0.66, p=0.01)
- Longer prior duration of ART OR 0.75 per year (95%CI 0.57-0.98, p=0.04)
- White Ethnicity OR 0.17 (95%CI 0.04-0.69, p=0.01)
Factors considered: randomization group, age, sex, risk group, ethnicity, baseline CD4, nadir CD4, CDC status, baseline limb fat, baseline thymidine analogue, the number of antiretrovirals previously received, time on therapy, weight at randomization and use of PI or NNRTI as the third agent in the regimen.
Author Summary:
- TDF and ABC similarly allow restoration of limb fat over 48 weeks when switching from thymidine analogues in persons with lipoatrophy
- Use of Zidovudine at baseline was consistently associated with lower fat mass recovery and an 85% lower chance of recovering >710g of limb fat over 48 weeks relative to d4T use at baseline
- Other factors associated with less fat recovery may include age, white race and longer duration of ART
- These data suggest early modifi cation of ART away from thymidine analogue based therapy should be considered
|
|
| |
| |
|
 |
 |
|
|