 |
 |
 |
| |
Resistance in EFV/TDF/FTC Study 934
|
| |
| |
"Lack of Resistance to Tenofovir DF at Week 48 and Impact of Baseline NNRTI Resistance Mutations on Treatment Response in Study 934"
Reported by Jules Levin
10th European AIDS Conference (EACS)
November 17-20, 2005
Dublin, Ireland
Poster Number PE7.3/16
DJ McColl, NA Margot, B Lu, AK Cheng, and MD Miller
for the Study 934 Team
Gilead Sciences, Inc., Foster City, California, USA
Study GS-01-934 is an open-label, randomized study comparing tenofovir DF (TDF), emtricitabine (FTC), and efavirenz (EFV) to lamivudine/zidovudine (3TC/AZT or Combivir) and EFV in antiretroviral
therapy (ART)-naïve patients.
Study Objectives:
Characterize the development of resistance in ART-naïve patients treated with the combination of FTC + TDF + EFV.
Determine the prevalence and effects of baseline NNRTI- and NRTI associated resistance mutations in this population of patients.
AUTHOR CONCLUSIONS:
No patient in this study developed K65R by Week 48.
- Resistance to FTC + TDF + EFV developed infrequently (4% of treated patients) and was primarily associated with NNRTI-resistance development.
- By Week 48, M184V/I developed less frequently on FTC + TDF + EFV (1%) compared to Combivir + EFV (3%) but this did not achieve statistical significance (p > 0.05).
- Primary NNRTI-resistance was present at baseline in 4.3% of ART-naïve patients in Study 934 and was significantly associated (p < 0.001) with risk of treatment failure on efavirenz-containing regimens.
- Baseline NRTI-resistance (TAMs, 2.7%) or non-B HIV-1 subtypes (5.5%) were not associated with reduced treatment responses.
- Baseline genotyping appears warranted when initiating NNRTI-based regimens in ART-naïve patients.
- The once-daily regimen of FTC + TDF + EFV showed superior efficacy compared to the twice-daily regimen of Combivir + EFV (84% vs 73% <400 c/ml).
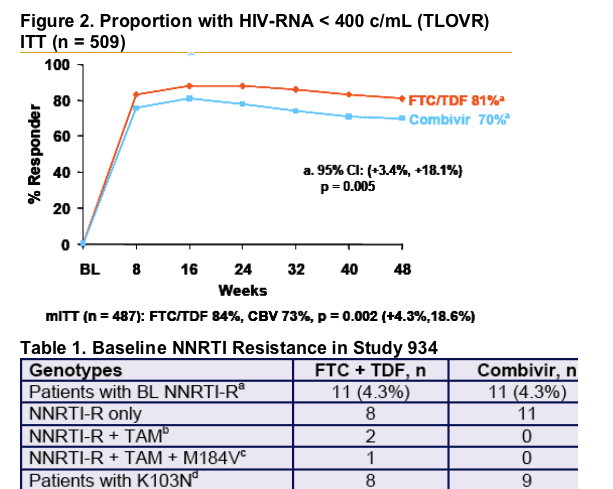
a. Patients (n = 22) came from 16 study sites in USA and EU with no geographic localization. Mean phenotypic susceptibilities to EFV, tenofovir, FTC, ZDV and 3TC at baseline were 28.6-fold reduced, 0.9-fold, > 4-fold reduced, 1.0-fold, and > 4-fold reduced (relative to wild-type), respectively. For patients without M184V at baseline (n = 21), mean phenotypic susceptibility to FTC and 3TC was 0.85-fold and 0.87-fold of wild-type, respectively. Three patients also had evidence of genotypic or phenotypic resistance to protease inhibitors.
b. Thymidine analogue mutations detected at baseline included M41L, D67N, K70R, K219Q, T215L/S.
c. No other patient entered the study with M184V/I and no patient had the K65R mutation at baseline.
d. K103N detected in 17 of 22 patients; other mutations included K101E, K103R, Y181C, Y188F, G190A/E.
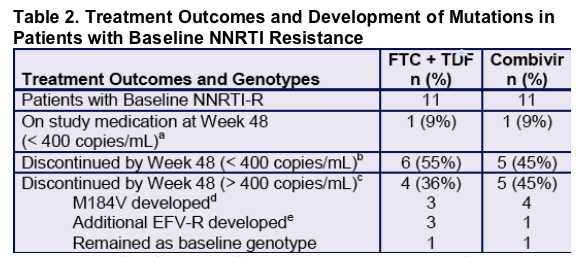
a. Two patients (8.4-fold and 29-fold reduced susceptibility to EFV) achieved < 50 copies/mL on study medication by Week 48.
b. Patients were lost to follow-up or switched medication due to baseline NNRTI resistance but were < 400 copies/mL at their last time point on study and were not genotyped.
c. Patients had confirmed > 400 copies/mL of HIV1 RNA while on study medication or after treatment switch and were genotyped. Compared to the mITT population, a significantly greater proportion of patients with baseline NNRTI-R (35/487, 7% vs 9/22, 41%) met resistance analysis criteria (p < 0.001, Fisher Exact Test).
d. No patient developed the K65R mutation. One FTC + TDF patient who had M41L and T215L mutations at baseline developed a mixture at codon 215 (T215C/F/R) with no effect on tenofovir susceptibility; the effect on FTC susceptibility could not be determined due to the concomitant development of the M184V mutation.
e. All four patients who developed more EFV-R mutations (L100I, V108I, Y188L, and P225H) also developed M184V.
Table 3. Patients with Baseline NRTI Resistance only (n = 13)
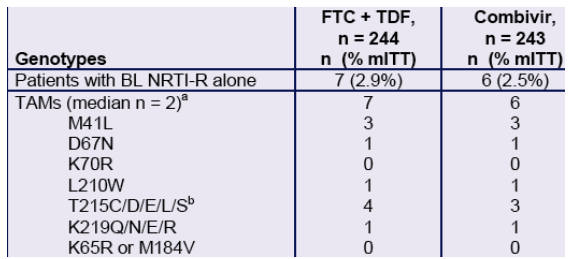
a. The maximal number of TAMs detected in a patient was three in a FTC + TDF patient who had M41L, L210W and T215C and 2.7-fold reduced susceptibility to ZDV at baseline (all other NRTIs susceptible). All thirteen patients were susceptible to the NRTI components of their respective regimens. Twelve of 13 were susceptible to EFV; one Combivir patient had low-level (2.9-fold) reduced susceptibility to EFV in the absence of detectable NNRTI-resistance mutations.
b. All T215 mutations were presumed reversions.
- By Week 48, all thirteen patients with baseline NRTI-resistance mutations had remained on study medication.
- 12/13 (92%) achieved < 400 copies/mL; 11/13 (85%) achieved < 50 copies/mL.
- 1 Combivir patient with baseline TAMs had virologic rebound.
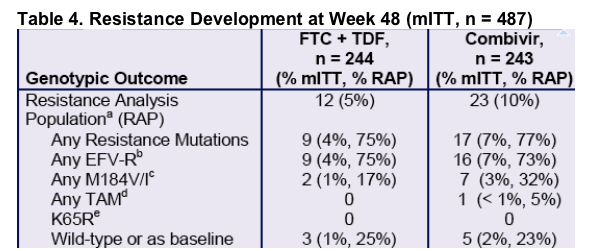
a. All patients with confirmed > 400 copies/mL of HIV RNA at Week 48 or early discontinuation analyzed. Patients with baseline NNRTI-resistance excluded (n = 22). Genotyping of 1 Combivir patient failed; % RAP for Combivir based on n = 22 with available genotype.
b. K103N developed in 21/25 patients; other NNRTI mutations that developed included K101E, K103E, V108I/M, V179D, Y188H, G190A/S, P225H, M230L.
c. Both FTC + TDF patients and 6/7 Combivir patients that developed M184V also developed EFV-R mutations.
d. TAM = thymidine analogue mutations: M41L, D67N, K70R, L210W, T215Y/F, K219Q/E/R/N; TAM detected was K70K/R.
e. In addition, no K65R developed among patients with baseline NNRTI resistance.
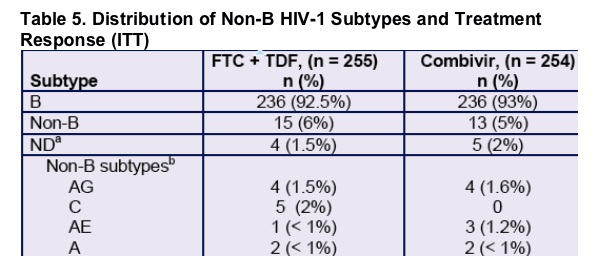
a. Sub-type could not be determined due to technical failure of baseline genotyping.
b. All others detected (BF, D, G and complex) were represented by ≤ 2 patients (< 1% of ITT population).
- All non-B HIV-1 isolates in both arms were considered wild-type at baseline.
- By TLOVR, 87% of FTC + TDF and 69% of Combivir patients with non-B HIV-1 were responders by Week 48 (p > 0.75 versus B subtypes).
- No FTC + TDF patients with non-B subtype HIV-1 isolates met resistance analysis criteria by Week 48.
- One Combivir patient (subtype AG isolate) met resistance analysis criteria and developed EFV-R + M184V.
BACKGROUND:
TDF selects for the K65R mutation in HIV-1 reverse transcriptase (RT) whereas FTC (and 3TC) select for the M184V/I mutation. Week 48 resistance analyses were conducted to determine the patterns and frequencies of mutations developing in HIV-1 from patients taking FTC + TDF + EFV.
Transmitted HIV-1 drug resistance is a potential limitation to initiation of ART in treatment-naïve patients but little data exists on the impact of transmitted drug resistance on treatment outcomes. Baseline genotyping of all patients in Study 934 enabled identification of patients with transmitted resistance and analysis of the impact of transmitted resistance on treatment response and resistance development.
METHODS:
- All genotyping and phenotyping of patient plasma HIV-1 was conducted at ViroLogic, Inc. (South San Francisco).
- All Study 934 patients were genotyped at baseline following study enrollment and therapy initiation.
- For patients with baseline NNRTI-resistance (n = 22), the DSMB & FDA recommended they be excluded from the primary endpoint analysis.
- Investigators were notified if affected.
- NNRTI-R patients were excluded from the modified ITT population (mITT, n = 487).
- Two populations were analyzed for efficacy results, mITT and ITT.
- Post-baseline genotyping and phenotyping was performed on all patients (both mITT and ITT) meeting resistance analysis (RA) criteria.
- All patients with confirmed > 400 copies/mL plasma HIV-1 RNA at Week 48 or early study discontinuation.
- Median time from failure to analysis - 14 weeks.
|
|
| |
| |
|
 |
 |
|
|