 |
 |
 |
| |
FTC Had Less M184 than 3TC
|
| |
| |
Pooled Analysis of Recent Emtricitabine and
Lamivudine Clinical Trials Reveals Differences
in Rates of Development of the M184V/I Mutation
Reported by Jules Levin
10th European AIDS Conference (EACS)
November 17-20, 2005
Dublin, Ireland
Poster Number PE7.3/17
DJ McColl1, B Lu1, MD Miller1, and K Borroto-Esoda2
1Gilead Sciences, Inc., Foster City, California, USA
2Gilead Sciences, Inc., Durham, North Carolina, USA
STUDY OBJECTIVE:
Characterize the frequency of development of the M184V/I mutation in patients experiencing VF on regimens containing either FTC or 3TC combined with another NRTI and the NNRTI efavirenz
Author Conclusions:
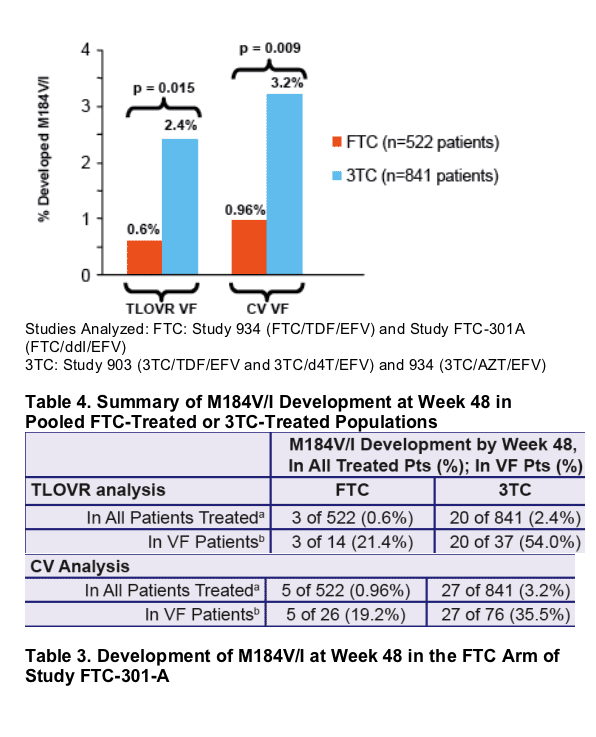
A pooled analysis of three recent phase III studies in treatment-naive patients using either FTC QD (n = 522 patients treated) or 3TC BID (n = 841 patients treated) combined with another NRTI and EFV, demonstrated a significantly lower rate of development of the M184V/I mutation in patients treated with FTC QD.
All clinical studies with FTC have investigated QD dosing. 3TC was originally approved for BID dosing and is currently approved for use as a QD agent, both singly and in fi xed dose combinations. Whether use of QD 3TC further increases the rate of M184V/I development relative to FTC is unclear.
Figure 1. Reduced Incidence of M184V/I in Patients Treated
with FTC vs 3TC in 3 Pooled Phase III Clinical Trials
INTRODUCTION
- Emtricitabine (FTC) and lamivudine (3TC) are nucleoside reverse transcriptase inhibitors (NRTIs) used as antiretroviral therapy (ART) for the treatment of HIV-1 infection
- Both FTC and 3TC are deoxycytidine analogues that are activated inside cells to their tri-phosphate (TP) forms. FTC-TP and 3TC-TP act as competitive substrate inhibitors of HIV-1 reverse transcriptase (RT)
- Both drugs can cause the selection of the M184V/I mutation in RT, resulting in high-level resistance to both drugs, and low-level resistance to abacavir
BACKGROUND
- Relative to 3TC, FTC is 4-10 fold more potent against laboratory and primary clinical isolates of HIV-11-3 and has both a longer plasma half-life (10 hours versus 7.9 hours4,5) and longer intracellular half-life (> 39 hours versus 16 hours4,6) in patient PBMCs
- These differences may influence the frequency of development of the M184V/I mutation in HIV-1 patients utilizing FTC compared to 3TC
- The frequency of M184V/I in patients experiencing virologic failure (VF) was assessed in recent large phase III clinical trials investigating FTC or 3TC combined with other NRTIs and the non-nucleoside inhibitor (NNRTI) efavirenz
METHODS:
- Phase III studies in ART-naive HIV-1 infected patients (pts) used in this analysis included:
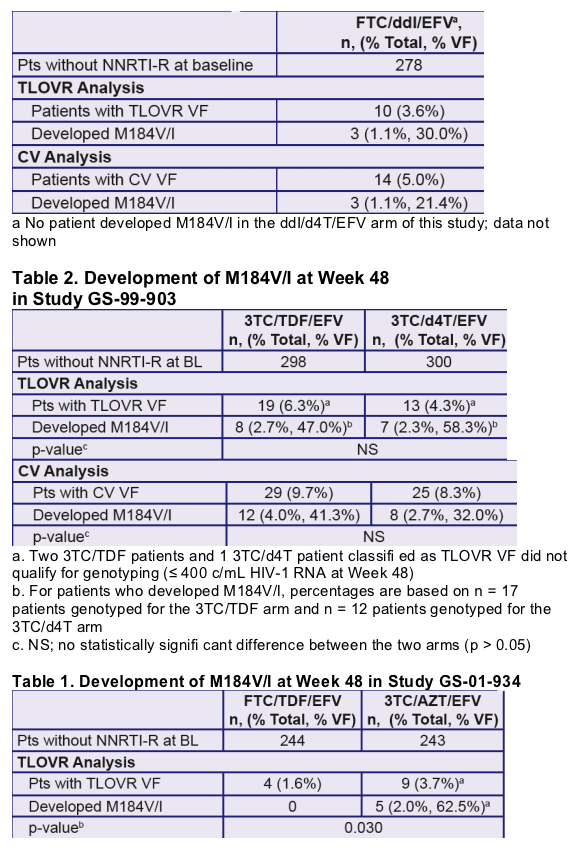
- FTC-301A (FTC + didanosine (ddI) + EFV vs stavudine (d4T) + ddI + EFV)
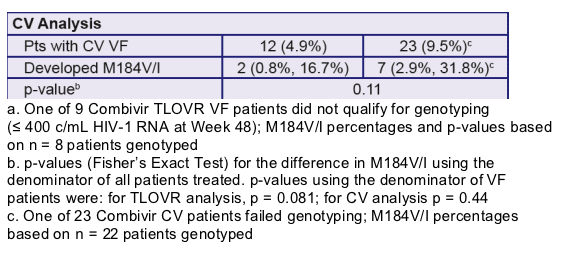
- GS-99-903 (3TC + tenofovir DF (TDF) + EFV vs 3TC + d4T +EFV)
- GS-01-934 (FTC +TDF + EFV vs 3TC + zidovudine (AZT) +EFV)
- In all studies analyzed, FTC was used QD and 3TC was used BID
- Patient groups were classified as VF according to either:
- FDA-defined "time to loss of virological response" (TLOVR) algorithm; includes VF patients classifi ed as rebound, never suppressed or added an ARV
- Presence of confi rmed > 400 copies/mL of plasma HIV-1 RNA at Week 48 or early study discontinuation, (clinical virology algorithm, CV); includes all TLOVR VF patients and those failing with adverse events, lost-to follow up or other reasons
- Patients with confirmed primary NNRTI resistance mutations (as defined by the IAS 2005 guidelines) at baseline in all three studies were excluded from the analysis
- Patients experiencing VF were genotyped using standard population sequencing procedures
- Genotypic data for all VF patients in FTC-containing trials were pooled and compared to the equivalent data from pooled 3TCcontaining trials; differences were analyzed for statistical significance using Fisher’s Exact Test
References
1. RF Schinazi, et al. AAC 1992; 36:2423-2431.
2. RF Schinazi, et al, AAC, 1993; 37:875-881.
3. M Tisdale, et al. Proc. Natl. Acad. Sci. USA 1993; 90: 5363-5656.
4. LH Wang, et al. AIDS Res. Hum. Retroviruses 2004, 20: 1173-1182.
5. R Bruno, et al. Clin Pharmacokinet. 2001, 40:695-700.
6. KHP Moore, et al. AIDS 1999, 13: 2239-2250.
|
|
| |
| |
|
 |
 |
|
|