 |
 |
 |
| |
MK-0518, the first Integrase Inhibitor for HIV, No Blarney
|
| |
| |
Reported by Jules Levin
European AIDS Conference (EACS)
Dublin, Nov 18, 2005
Today was groundbreaking and exciting at the European AIDS Conference, as the initial study results, phase II, were presented for the Merck integrase inhibitor MK-0518, and week 24 study results for TMC-125, a new NNRTI for patients with resistance to efavirenz and nevirapine. This report will provide study data reported for the integrase inhibitor, and a report will follow regarding TMC-125.
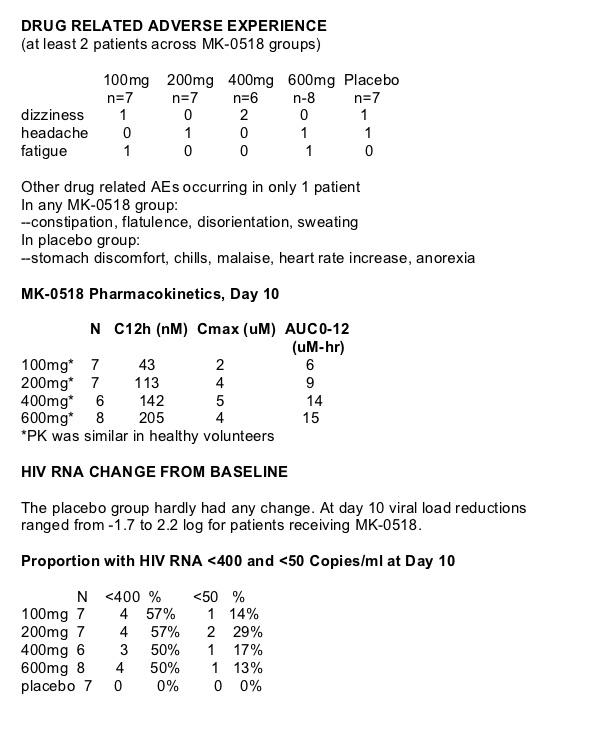
28 treatment-naive patients received 10 days of monotherapy with MK-0518 and 7 study participants received placebo. HIV viral load reductions were a potent -1.7 to 2.2 log for the various doses of the integrase inhibitor administered. 50-57% of patients receiving MK-0518 achieved <400 copies/ml and 13-29% achieved <50 copies/ml viral load. The drug appeared safe and well tolerated, safety data reviewed below but there were no serious adverse events & no discontinuations due to adverse events. . Based on these results, a 48-week dose-ranging trial of MK-0518 versus efavirenz - a non-nucleoside reverse transcriptase inhibitor - in combination therapy has been initiated in treatment-naive patients.
"Antiviretroviral effect of MK-0518, a Novel HIV-1 Integrase Inhibitor, in ART-Naive HIV-Infected Patients"
J.O. Morales-Ramirez reported the study results in a morning oral session in Dublin.
BACKGROUND
There is urgent need for ART against new targets, as many patients have resistance to the currently available drug targets. MK-0518 has displayed potency in vitro (in the test tube):
--IC95=33±23 nM in 50% NHS; IC50 10 Nm
--In vitro synergy with licensed ARTs
--Active against HIV isolates resistant to current ARTs
--HIV isolates resistant to MK-0518 remain sensitive to other ART classes
--Preclinical animal studies to 12 months show no substantive issues
--Phase I data supported doses of 100 to 600 mg in HIV+ patients:
excellent tolerability at 100mg twice daily (bid) oral dosing, C-12hr>IC95, and no significant food effect
STUDY DESIGN
Randomized, double-blind, placebo-controlled study of MK-0518 vs placebo in HIV+ ART-naive patients.
Study regimen:
--dose: 100mg, 200mg, 400mg, 600mg p.o. or placebo all bid (every 12 hours).
--duration: 10 days monotherapy
Endpoints of study:
--HIV RNA (viral load0 & CD4 counts
Patients were required to have >5000 copies/ml HIV RNA & Cd4 count >100, and near normal safety laboratory values. Patients were excluded for immunosuppressive therapy, acute hepatitis, chronic liver disease, and renal disease
BASELINE CHARACTERISTICS
35 patients enrolled from US, Canada, Australia
Age- 36-46 yrs
Mostly males 86-100%
40-50% non-white
HIV RNA: 34,000 (4.53 log) to 99,000 copies/ml (4.97 log)
Mean CD4 count: 256-569
% with AIDS: 13-17%
SAFETY
Clinical adverse experience (AE)- generally mild & transient.
Lab AE - one patient with increased ALT in any MK-0518 group
--grade 1 elevation at day 5 resolved by day 10 and did not require treatment interruption
No dose related toxicities.
No serious adverse events.
No grade 3 or 4 laboratory toxicity (per protocol criteria).
No discontinuations due to AE.
|
|
| |
| |
|
 |
 |
|
|