 |
 |
 |
| |
Pegasys/RBV Retreatment for IFN/RBV Failures
|
| |
| |
Reported by Jules Levin
Over the weekend I sent a report out to you with the preliminary results from the EPIC Study, which examines re-treatment with PegIntron plus RBV in patients previously treated with interferon+ribavirin. Poynard reported a preliminary SVR of 21%. Also at EASL April 2005, a poster reported results from an Canadian open-label Pegasys Expanded Access Program. This poster reports a 23% SVR rate for patients who previously failed interferon+ribavirin and only 800mg per day of ribavirin was used rather than the recommended 1000 or 1200mg. However, you are not supposed to compare results from different studies, particularly because often the baseline characteristics of patients very between studies & this affects the outcomes.
"Peginterferon alfa-2a (40D) Pegasys) plus ribavirin (Copegus) in chronic hepatitis C patients wjo failed previous interferon based therapy: results of a multicenter open-label expanded access programme in Canada"
M Sherman & colleagues reported these study results & information at the 40th EASl in Paris in a poster.
INTRODUCTION
In randomized, multicenter phase III studies, overall SVR rates of up to 63% have been achieved in patients treated with Pegasys plus Copegus (ribavirin): 46-52% in HCV genotype 1 & 76-84% in HCV genotype 2 or 3 patients.
The management of patients who have relapsed or failed to respond to previous interferon-based therapy is an important, but as yet unresolved, issue in the management of chronic hepatitis.
This study evaluates the efficacy & tolerability of Pegasys plus ribavirin in relapsers & nonresponders enrolled in the Canadiam multicenter expanded access program.
STUDY DESIGN
The study patients were those who relapsed or who failed to respond to previous conventional interferon. Patients were assigned at their physicianÕs discretion to receive combinatiob therapy with Pegasys+RBV 800mg per day for 24 or 48 weeks.. SVR was defined as undetectable HCV RNA (<50 IU/mL by Cobas Amplicor HCV Test, v2.0) at the end of a 24-week untreated follow-up period ( week 48 in the 24-week treatment group & week 72 in the 48-week treatment group). A total of 361 patients were enrolled. 355 patients were assigned to combination therapy & 6 patients were assigned Pegasys monotherapy. Analyses were conducted using the intent-to-treat (ITT) population.
RESULTS
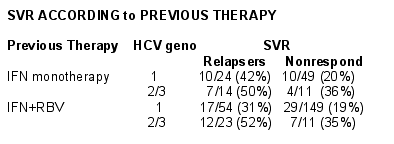
Patients assigned to combination therapy had received previous interferon-based therapy (119 relapsers & 236 non-responders). Of these 355 patients, 253 individuals (71%) had received previous IFN plus RBV (67% of relapsers & 73% of non-responders).
Baseline characteristics were generally similar between relapsers & non-responders, although a higher proportion of non-responders than relapsers had HCV genotype 1 (84% vs 65%).
GENOTYPE 1: 65% relapsers; 84% nonresponders
70% males
age: 47 yrs
88% Caucasian
weight: 80 kg
BMI: 27 kg/m2
HCV RNA: 32-37% >850,000 IU/mL; 23-27% 500,000 to 850,000 IU/mL; 40% <500,000
IU/ml
No cirrhosis: 50%
Transition to cirrhosis (F3): 21-23%
Cirrhosis (F4): 23-29%
75% of the nonresponders had previous therapy with IFN/RBV & 67% of relapsers hadIFN/RBV therapy.
RESULTS
Overall, 48/119 (40%) relapsers & 54/236 (23%) non-responders achieved an SVR following treatment with pegasys plus ribavirin.
NON-RESPONDERS
All genotypes: 23%
Genotype 1: 20%
Genotype 2/3: 35%
RELAPSERS
All genotypes: 40%
Genotype 1: 35%
Genotype 2/3: 51%
SAFETY
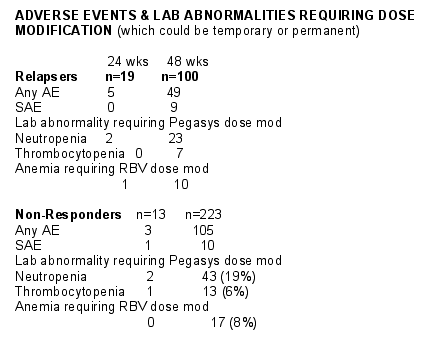
The overall incidence of serious adverse events was similar in non-responders & relapsers. A total of 12 adverse events were reported in 11 of the 236 non-responders (5%) & a total of 14 adverse events were reported in 9 of 119 relapsers (8%). Overall, 10 events occurring 9 patients were judged to be related to study treatment by the investigators.
Similar proportions of relapsers & responders receiving 48 wks of therapy requiring peginterferon required Pegasys dose modifications for neutropenis (23% vs 19%), or thrombocytopenis (7% vs 6%) & RBV dose mod modifications for anemia (10% vs 8%).
|
|
| |
| |
| |
|
 |
 |
|
|