 |
 |
 |
| |
Pegasys for HBV, 72 Weeks: HBsAg Seroconversion Response
|
| |
| |
"SUSTAINED HBSAG SEROCONVERSION IN PATIENTS WITH CHRONIC HEPATITIS B TREATED WITH PEGINTERFERON a-2a (40 kDa) (PEGASYS)"
S. Hadziyannis1, G.K.K. Lau2, P. Marcellin3, T. Piratvisuth4, G. Cooksley5, F. Bonino6, A. Chutaputti7, M. Diago8, R. Jin9, N. Pluck10
1 Department of Medicine and Hepatology, Henry Dunant Hospital, Athens, Greece
2 Department of Medicine, Queen Mary Hospital, University of Hong Kong, Hong Kong, China
3 Service d'Hepatologie, INSERM Unite 481 and Centre de Recherches Claude Bernard sur les Hepatites Virales, H™pital Beaujon, Clichy, France
4 Department of Medicine, Songklanakarin Hospital, Songkla, Thailand
5 Clinical Research Department, Royal Brisbane Hospital, Brisbane, Australia
6 IRCCS, Ospedale Maggiore di Milano Policlinico, Milan, Italy
7 Department of Medicine, Pramongkutklao Hospital, Bangkok, Thailand
8 Seccion de Hepatologia, Hospital General Universitario de Valencia, Valencia, Spain
9 Digestive Disease Department, Beijing You An Hospital, Beijing, China
10 Roche, Welwyn, UK
Here is link to 1 year followup data from this study in HBeAg-negative reported at EASL:
SUSTAINED RESPONSE TO PEGASYS IN HBeAg-NEGATIVE CHRONIC HEPATITIS B: 1-YEAR FOLLOW-UP DATA FROM A LARGE, RANDOMISED MULTINATIONAL STUDY
http://www.natap.org/2005/EASL/easl_18.htm
Background: HBsAg seroconversion (loss of HBsAg and appearance of anti-HBs) is considered to be the ultimate goal of anti-HBV treatment. It is, however, a rare event occurring in a negligible number of patients treated with nucleos(t)ide analogues, mostly in patients with HBV genotype A and after >1 year of continuous therapy.
Objectives: To describe HBsAg responses in 1351 patients with HBeAg-positive or -negative CHB who were enrolled in two randomised, partially double-blind, multinational studies comparing peginterferon a-2a (40 kDa) (PEGASYS), peginterferon a-2a plus lamivudine, and lamivudine alone.
Methods: HBeAg-positive (n = 814) and -negative (n = 537) patients received (1:1:1) either peginterferon a-2a (180 µg once-weekly) + placebo, peginterferon a-2a + lamivudine (100 mg once-daily) or lamivudine. Patients were treated for 48 weeks and assessed 24 weeks after the end of treatment (week 72). Patients were considered to have sustained HBsAg seroconversion only if they were HBsAg-negative/anti-HBs-positive at week 72.
Results: Over 75% of patients in the studies were of Asian origin; predominant HBV genotypes in the study populations were B (27%) and C (50%). Overall HBsAg seroconversion rates at week 72, and rates stratified by ethnicity and genotype are shown in the Table. HBsAg seroconversion only occurred in patients achieving HBeAg seroconversion - among responders with HBeAg seroconversion, the rate of HBsAg seroconversion was 10% with peginterferon a-2a-containing therapy and 0% with lamivudine.
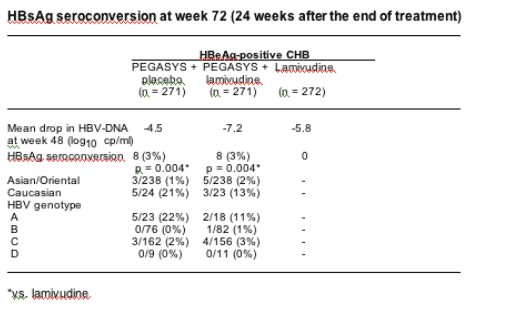
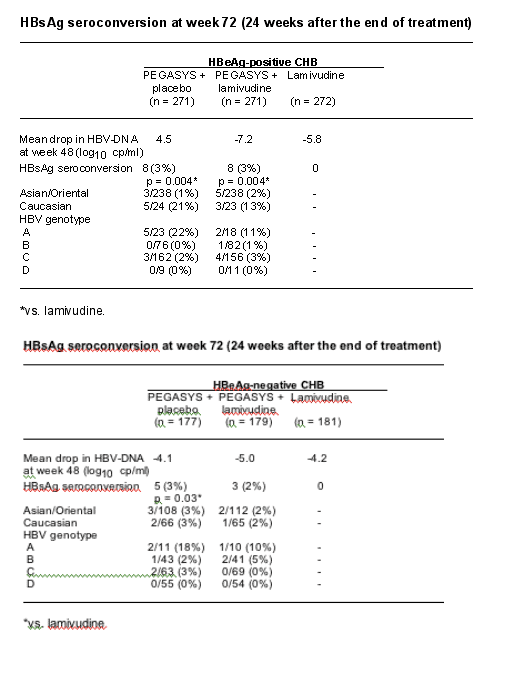
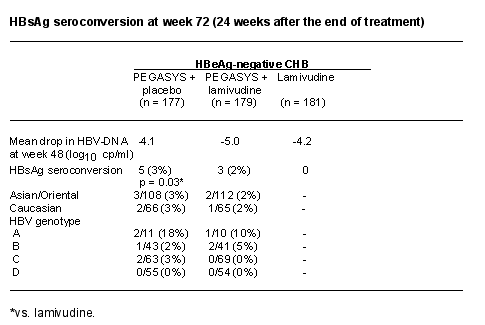
Conclusions: In our study, patients treated with a peginterferon a-2a-containing regimen for a defined duration achieved sustained HBsAg seroconversion, while patients treated with lamivudine did not. The magnitude of HBV-DNA suppression on treatment does not seem to be the major factor leading to HBsAg response, but rather the additional immunomodulatory effect associated with peginterferon a-2a (40 kDa) (PEGASYS) treatment.
|
|
| |
| |
| |
|
 |
 |
|
|