 |
 |
 |
| |
NM-283 New HCV Drug, new 24 week data; New Developments in HCV at EASL
|
| |
| |
NM283 is potentially the most timely & important development at this time in treatment for patients who were partial responders to PegIFN/ribavirin & perhaps non-responders. Two HCV protease inhibitors are being studied in patients but these studies are in earlier stage of development than NM283.
The final session at EASL (40th annual meeting of the European association for the study of liver diseases) has just been completed at 12:30pm Paris time. The final oral session was all on HCV & there were several significant study results reported. NM283 is a new drug for HCV treatment, a polymerase inhibitor. It is the first new HCV drug to advance this far into study development aside from Viramidine. NM283 study results were reported for a phase II study this morning.. Further studies are being planned. I report below the study results reported here at EASL. In this final HCV oral session, study results were reported for Viramidine, a potential substitute for ribavirin, because Viramidine has much less risk for anemia associated with its use. In addition, there were two oral presentations regarding peginterferon use as a maintenance therapy to delay the advancement of HCV disease progression. The preliminary results from the Co-Pilot Study reported here in this session by Nezam Afdahl showed benefits to use of peginterferon as a maintenance therapy. The results are preliminary as the study is still in progress. Afdahl showed beneficial effects on the development of portal hypertension with the use of PegIntron compared to the control arm of the study. These findings are preliminary but show promise. It remains unsure if the benefit was due to reduced HCV RNA from the PegIntron or a results of an effect of PegIntron on the portal hypertension, Afdahl said in his presentation. Finally, Thierre Poynard presented early results from the EPIC Study, which is also a maintenance therapy study. He only presented SVR data, the study is ongoing & looking at the effect of pegIFN in slowing disease progression. I will report the results of these other studies to you following this report on NM283.
NM283
"Enhanced Antiviral Efficacy for Valopicitabine (NM283) Plus Peg-Interferon In Hepatitis C Patients With HCV Genotype-1 Infection:
Results of a Phase IIa Multicenter Trial"
N Afdhal, M Rodriguez-Torres, E Lawitz, E Godofsky, G Chao, B Fielman, S Knox, N Brown
1Department of Medicine, Beth Israel Deaconess Medical Center, Boston, MA, USA. 2Fundacion de Investigacion de Diego, Santurce, PR, USA. 3Alamo Medical Research, San Antonio, TX, USA. 4Bach & Godofsky, Bradenton, FL, USA. 5Idenix Pharmaceuticals, Cambridge, MA, USA
Nezam Afdahl orally presented this information & data today April 17 in Paris at EASL. Following the report on NM283 phase II study results are two additional posters presented at EASL on NM283 PK & an in vitro study.
AUTHOR CONCLUSIONS:
Marked antiviral activity for valopicitabine + peg-IFN_
--Proportion of PCR-neg patients at Weeks 12 and 24 appears better than peg-IFN_-2b alone by historical comparison
For 9 ComboRx patients completing Week 24:
--Mean HCV RNA decline of 4.5 log10 IU/mL (range: -2.33 to -6.2 log10)
--9 of 9 pts have achieved at least 2 log10 decrease from Baseline:
8 of 9 patients below LLOQ for Amplicor PCR (< 600 IU/mL)
6 of 9 patients below LLOD for Taqman PCR (<10 IU/mL)
Continuing 'late' HCV RNA reductions:
--4 of 5 pts with detectable HCV RNA at Week 12 had subsequent multi-log drops by Wk 24
Kinetics of response to Valopicitabine + Peg-IFN_ may differ from kinetics of response to peg-IFNa + RBV
No HCV RNA 'breakthroughs' to date
--viral genotying (HCV Pol) underway)
--good tolerability
negligible hemotologic side effects, possible safety advantage
--larger Phase IIb trials in prior treatment failures & treatment-na•ve patients underway
"NM283 may offer improved efficacy and safety especially for HCV 1 genotype patients & prior treatment failures"
INTRODUCTION and BACKGROUND
Peg-IFNa-2b monotherapy has 25% ETR (week 48) and 5-8% SVR in HCV-1 genotype patients.
Addition of ribavirin to Peg-IFNa-2b increases SVR to 42% in HCV-1 genotype patients.
Valopicitabine (NM283) reduced HCV RNA in a phase I/II trial:
--mean 1.2 log (94%) reduction in HCV RNA in two weeks at optimal valopicitabine dosing levels.
--87% of patients had previously failed IFN-based therapies.
Valopicitabine and IFNa show synergistic antiviral effects in BVDV model in vitro
--supports early clinical investigation of valopicitabine plus IFNa, to maximize antiviral efficacy and minimize resistance. (see abstract at end of this report).
INITIAL STUDY DESIGN
Valopicitabine Phase IIa Trial
Safety, antiviral activity, and pharmacokinetics of valopicitabine versus valopicitabine + peg-IFNa-2b during 28 days treatment:
--open-label 'PK interaction' and safety study
--30 treatment-na•ve adults with compensated chronic hepatitis C (CHC)
--HCV-1 genotype, HCV RNA >5 log IU/mL, ALT <5 x ULN
--randomized 2:3 to valopicitabine (n=12) vs valopicitabine + peg-IFNa (n=18)
--peg-IFNa-2b: 1.0 ug/kg given weekly (days 8, 15, and 22)
--valopicitabine: dose escalation starting with 400 to 600 to 800 mg/day to day 8, then 800 mg/day to day 28
Afdahl described that the early data was encouraging so treatment was extended to 12, then 24, and finally to 48 weeks via protocol amendments, with FDA & investigator agreement. Virologic response & tolerability at week 12 determined eligibility for extended treatment. Study is ongoing to one year: 12 & 24 week datas today is being presented.
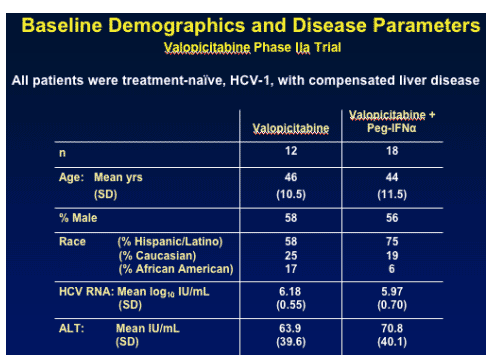
BASELINE CHARACTERISTICS & DISEASE PARAMETERS
Baseline viral load (HCV RNA) was 6.18 log IU/mL for patients receiving Valopicitabine & 5.97 log IU/mL for patients receiving NM283+ Peg-IFNa. ALT was 63-70 IU/mL. Interestingly, 58-75% of patients in the study were "Hispanic/Latino) due to enrollment in Puerto Rico. Of note, Afdahl characterized these patients as having African background suggesting this ethnic relationship to evaluation of response to therapy. 25-19% were Caucasian; 17-6% were African-American.
|
|
 |
| |
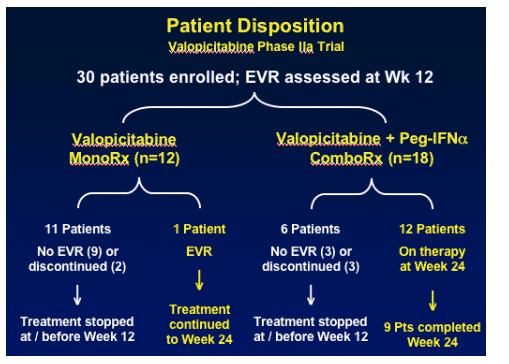
For the 30 patients enrolled, Early Viral response (EVR) was assessed. 11/12 patients on NM283 monotherapy did not achieve EVR. Of the 18 patients receiving combination therapy, for 6 patients treatment was stopped at or before week 12, 3 no EVR, or discontinued (3). 12 patients were on combination therapy at week 245, 9 patients completed week 24.
|
|
 |
| |
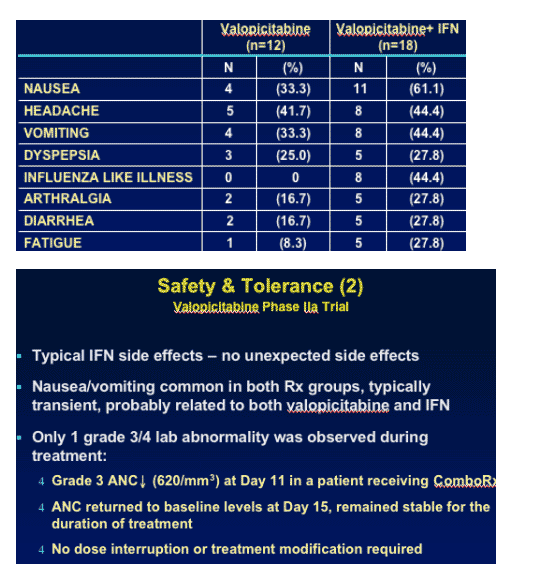
Safety & Tolerance
Valopicitabine Phase IIa Trial
--Clinical safety satisfactory overall
--No serious adverse events or dose-limiting toxicities
--Most frequent adverse events, regardless of attributability to study drug:
NM283 is associated with gastrointestinal upset, thus nausea, diarrhea, vomiting can occur in the first few days after starting therapy. But in talking with company officials I've been told these side effects pass quickly.
|
|
 |
| |
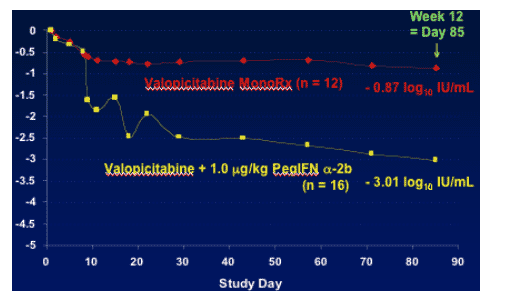
Week 12 Viral Response
Valopicitabine Phase IIa Trial
All 28 Patients with Data Past Week 1
Mean serum HCV RNA change from baseline:
-3.01 log IU/mL: NM283+PegIFNa-2b comnination (n=16)
NM283 monotherapy (n=12): -0.87 log IU/mL
Combo treatment at week 12: 12 patients with >1.7 log reduction; 4 PCR negative.
|
|
 |
| |
Week 24 Viral Response
Valopicitabine Phase IIa Trial
All 9 ComboRx Patients & 1 MonoRx Patient Completing Week 24
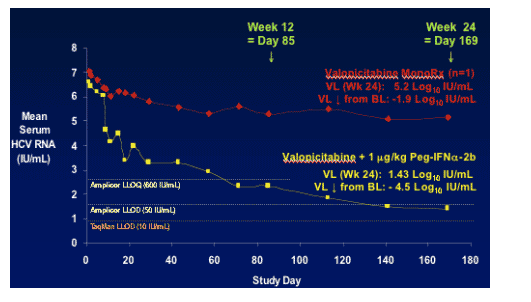
NM283 had viral load reduction of -1.9 log IU/mL from baseline for the 1 patient on monotherapy.
NM283+PegIFNa-2b combination therapy resulted in mean HCV RNA reduction from baseline of -4.5 log IU/mL.
I was told by investigators that maximal viral load reduction was 6.2 log IU/mL from baseline. Baseline viral load was 6 log IU/mL, about 3 million copies/ml. You can see in the graph below that the mean viral load reduction was below the lower limit of quantification of the Amplicor assay using 50 Iu/mL as a cutoff. From the graph below of the individual patient responses you can see that 6/9 patients achieved viral load reductions below the most sensitive assay used: LLOD Taqman (10 IU/mL). 7/9 were below LLOD Amplicor 50 IU/mL. 8/9 were below LLOD Amplicor 600 IU/mL.
|
|
 |
| |
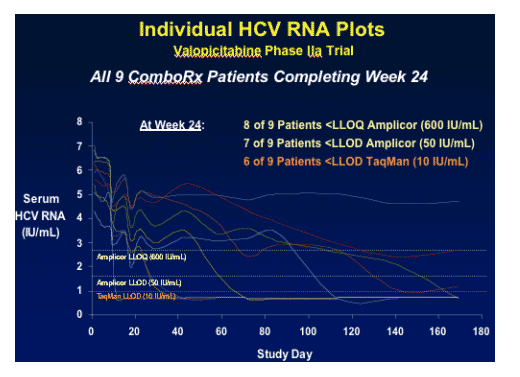
You can see from the graph below that 3 patients had viral load at baseline between 6-7 logs and 1 of the 3 had a maximal reduction. Of course, this is a small study so we will have to look for results from larger studies to evaluate more fully the viral load reductions. But this initial study shows promising results, particularly for patients who were previous partial responders & need an extra viral load push from a new class of HCV drug, polymerase inhibitor.
|
|
 |
| |
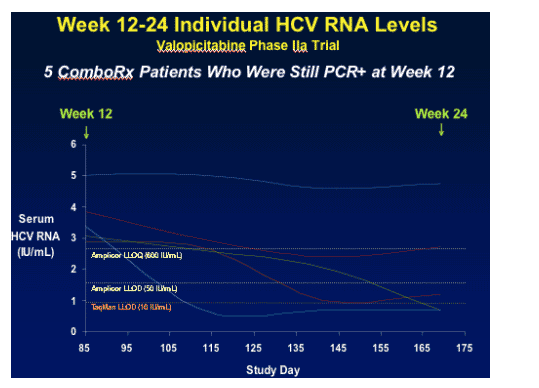
The graph immediately below displays the viral load responses of the 5 combination therapy patients who were still PCR+ at week 12. As you can see 3 of these patients had viral load reduced below 50 IU/mL & 1 patient achieved <10 IU/mL. 1 Patient was at 600 IU/mL & the 5th did not achieve any reduction.
|
|
 |
| |
PHARMACOKINETICS AND PHARMACODYNAMICS OF VALOPICITABINE (NM283), A NEW NUCLEOSIDE HCV POLYMERASE INHIBITOR: RESULTS FROM A PHASE I/II DOSE-ESCALATION TRIAL IN PATIENTS WITH HCV-1 INFECTION
X.J. Zhou1, N. Afdhal2, E. Godofsky3, J. Dienstag4, V. Rustgi5, L. Schick6, D. McInery7, B.A. Fielman1, N.A. Brown1
1 Idenix Pharmaceuticals, Inc, Cambridge, MA, USA. 2Beth Israel Deaconess Medical Center, Boston, MA, USA. 3Bach & Godofsky, Bradenton, FL, USA. 4Massachusetts General Hospital, Boston, MA, USA. 5Metropolitan Research, Fairfax, VA, USA. 6 University of Massachusetts Health Center, Worcester, MA, USA. 7 Northwest Medical Specialties, Tacoma, WA, USA
Background: Valopicitabine (NM283), an orally bioavailable valine prodrug of NM107, is a nucleoside analog exhibiting anti-HCV activity via direct inhibition of viral RNA polymerase. As a part of a first-in-man dose-escalation trial, we investigated NM283 pharmacokinetics (PK) and its pharmacodynamic (PD) implications.
Patients and Methods: Patients (12/dose cohort) were randomized 10:2 to treatment with NM283 (50-800 mg/day) or placebo for 15 days, with 14 days of follow-up. Assessments included safety, PK, and serum HCV RNA reduction. Patients were HCV-1-infected adults (18-65 yrs; 87% nonresponders to prior IFN-based therapies) with compensated, noncirrhotic chronic liver disease, ALT < 10 _ ULN and serum HCV RNA >5 log10 copies/ml by COBAS Amplicor PCR assay.
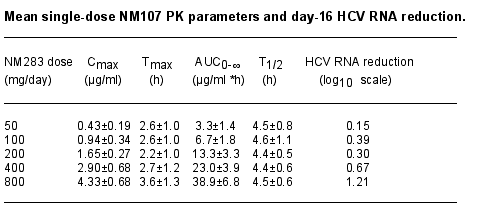
Results: After oral administration, NM283 was rapidly absorbed and extensively converted to NM107, the target moiety, and a minor metabolite, NM106. NM283 exhibited a short plasma half-life (T1/2) of ~1.4 h with comparable single dose and steady-state plasma exposures. No pre-dose or trough NM283 was detected. NM106 was only transiently detected at lower doses (50 and 100 mg), but became more consistently measurable with repeat daily dosing, and with higher doses. This metabolite exhibited a plasma T1/2 of ~12 h. NM107 was the major plasma species detected, representing ~80% of plasma exposure on a molar basis, followed by NM106 (~15%) and NM283 (<5%). Systemic exposure of NM107, NM106 and NM283 (AUC and Cmax) increased proportionally with NM283 dose. The AUC and Cmax of NM107 and NM106 correlated significantly with serum HCV RNA viral load reduction at day 16 (r > 0.7, P < 0.001), evidence of dose-related anti-HCV activity.
|
|
 |
| |
Conclusions: NM283 is efficiently absorbed and converted to NM107, the target moiety, upon oral administration with dose proportional plasma exposure. Higher plasma exposure is associated with better antiviral efficacy.
ENHANCED ANTIVIRAL ACTIVITY OF NM107, ALONE OR IN COMBINATION WITH INTERFERON
V. Bichko1, M. Tausek1, L. Qu1, M. LaColla1, S. Bergelson1, C. Pierra2, S. Benzaria2, D. Storer2, G. Gosselin2, J.P. Sommadossi1, D. Standring1
1 Idenix Pharmaceuticals Inc., Cambridge, MA, USA
2 Idenix SARL, Montpellier, France
Background: NM107 is 2c-C-methylcytosine, a ribonucleoside analogue that inhibits flavi- and pestivirus replication in vitro. NM283, an orally bioavailable prodrug of NM107, is currently in phase II clinical development for the treatment of chronic hepatitis C and was shown to reduce serum hepatitis C virus (HCV) RNA levels in both chronically infected chimpanzees and in patients with chronic hepatitis C. The objective of this study was to evaluate the antiviral activity of NM107 in vitro in combination with recombinant human interferons a-2b (Intron A) or b (Avonex).
Methods: The antiviral activities of NM107, interferon a-2b, interferon b and combinations thereof were studied in vitro using infection with bovine viral diarrhea virus (BVDV), a pestivirus related to HCV. Several virus strains from both cytopathic (cp) and noncytopathic (ncp) biotypes were used in de novo and/or persistently infected Madin-Darby bovine kidney (MDBK) cells.
Results: In vitro studies on BVDV NS5B polymerase indicate that NM107 triphosphate is a specific chain terminator of BVDV RNA synthesis. In cell-based assays, NM107 is a potent inhibitor of BVDV propagation (EC90 0.87±0.18 µM) and could eradicate a persistent BVDV infection after 28 days of treatment. Interferon a-2b inhibited BVDV modestly in vitro (EC90 32.5±18.2 IU/ml). Combination of 4 µM NM107 and 2000 IU/ml interferon a-2b exhibited synergistic antiviral activity. The reduction in virus titer was 4.56 log10, 2.4 log10 higher than the calculated logarithmic additive effect (2.16 log10). Enhancements of antiviral activity of over 4 log10 were seen for combinations of interferon a-2b and NM107 with the NY1 strain of BVDV. However interferon b did not inhibit BVDV and had no effect on the antiviral potency of NM107.
Conclusions: NM107 is a promising antiviral agent that acts synergistically with interferon a-2b in vitro. Clinical studies of NM283 in combination with interferon a are ongoing.
|
|
| |
| |
| |
|
 |
 |
|
|