I am in Paris at the 40th meeting for the European Association for the Study of Liver Diseases. I will be reporting to you on HCV & HBV information presented at this conference. Data on several new drugs in early development for HCV & HBV will be presented at this meeting. Jules Levin
Tipranavir appears to be a potent PI against protease inhibitor resistance. Approval from the FDA is expected very soon. In the meantime, the drug is available through the manufacturer’s Expanded Access Program. Speak to your doctor for information about the EAP.
This article reports information presented at the European Resistance Workshop on tipranavir, its potency and developing information on its resistance profile, particularly new information regarding phenotypic resistance cutoffs. This new resistance information helps to inform you about how to use tipranavir; under which resistance situations can the drug be effective and the degree of its effectiveness. For patients with extensive resistance to HIV drugs, adding tipranavir only to a regimen will not usually be effective. It's important to make sure you add new & effective drugs. Often T-20 (Fuzeon) is the only new drug that should have major potency for a patient with extensive resistance to HIV drugs. It can also be helpful to perform resistance testing to help evaluate which HIV drugs will be effective; as well, it's important to consider treatment history because resistance testing can have difficulty detecting drug resistance if you have not used a drug for a while.
Soon forthcoming articles on protease inhibitors at the Workshop will report on a comparison of ritonavir-boosted PIs in treatment-experienced patients, more on the resistance profile for atazanavir/r, the potency of Kaletra, and a report on the first evidence that two Gag mutations may have a big impact on virologic response to protease inhibitors.
"The 20 Faces of HIV"
European Resistance Workshop–Part 2. Protease Inhibitors
Athens, Greece, April 2005
Written for NATAP by Mark Mascolini (markmascolini@earthlink.net)
With tipranavir coming before the FDA’s Antiviral Drugs Advisory Panel in May, Boehringer Ingelheim’s Douglas Mayers made a timely appearance at the Third European HIV Drug Resistance Workshop to update attendees on the potential of this protease inhibitor (PI) against resistant virus.
But his was hardly the only useful survey of PI prowess against resistant virus at the March 30-April 1 workshop. Other investigators compared ritonavir-boosted regimens, with a special fix on atazanavir and lopinavir. Two studies explored the impact of mutations not in protease, but in the gene that encodes HIV’s Gag enzyme.
What will you get from tipranavir?
After years of development, tipranavir seems set to fulfill its early in vitro promise as a PI that reins in resistant virus better than current ritonavir-boosted options. Boehringer bosses are confident enough that they have already assigned the agent a brand name, Aptivus, in advance of a May 19 FDA review.
How apt that name may prove will be clear only when thousands of people with PI-resistant virus start taking this drug. At the 2005 Conference on Retroviruses, a 6-month comparison of 1100 PI-experienced people randomized to tipranavir/ritonavir or lopinavir/ritonavir found a significantly better response rate with tipranavir in a noncompleter-equals-failure analysis–39.6% versus 21.4% (P < 0.0001) [1]. When statisticians confined the analysis to lopinavir-naive people, tipranavir still yielded a better 6-month response (45.3% versus 36.1%), but the difference between arms was no longer significant.
Comparing tipranavir/ritonavir with four other boosted PIs, these researchers charted steeper viral load drops with tipranavir regardless of the mutation set studied–all changes from the protease consensus sequence; primary mutations at codons 30, 46, 48, 50, 82, 84, and 90; or substitutions at codons 33, 82, 84, or 90 [2].
But tipranavir may have one drawback for people with the most highly resistant virus: A study combining it with boosted lopinavir, amprenavir, or saquinavir charted 45%, 50%, and 80% lower trough concentrations of those PIs after people added tipranavir [3]. The CYP3A4-inducing effect of tipranavir appears to offset the inhibiting effect of ritonavir.
When will tipranavir work best? It’s still not easy to say, although Douglas Mayers and Boehringer scientists may be closing in on an answer. Ongoing work failed to confirm early hints that mutations at protease positions 33, 82, 84, and 90 form a compact set of danger signals for tipranavir.
Resistance to tipranavir turned out to be more complicated than that. Analyzing the impact of changes at all 99 protease positions on response to tipranavir in phase 2 and 3 trials, researchers compiled a list of 21 mutations at 16 positions that hobble tipranavir. For the record, Table 2.1 spells them out.
Table 2.1. Mutations in the tipranavir resistance score
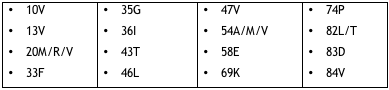
Some of these changes, Mayers observed at the workshop, don’t appear in mutation scores for any other PI–the unfamiliar wrinkles at positions 13, 35, 43, 58, 74, and 83. On the other hand, tipranavir apparently owes some of its muscle against more common resistant strains to the absence of D30N, G48V, N88D/S, and L90M from the resistance score.
L90M? Wasn’t that one of the fearsome foursome Boehringer once billboarded as potential tipranavir threats? That designation, Mayers explained, arose from an early Virco study that linked L90M to lowered viral susceptibility when combined with the keystone protease mutations V82T and I84V [4]. Other research spied L90M in clusters of critical mutations that robbed tipranavir of brawn. But Boehringer ultimately decided not to include it in tipranavir’s 21-item mutation score for three reasons:
- Tipranavir does not select L90M in vitro or in clinical isolates.
- Multivariate analysis of lowered susceptibility to tipranavir did not implicate L90M.
- Multivariate analysis of clinical responses to tipranavir did not tar L90M.
Mayers and colleagues concluded that L90M–combined with critical changes at codons 82 and 84–is merely a marker of highly mutated virus that bears a bevy of mutations from the tipranavir resistance score.
And what about that 21-item mutation score? Will genotypers and VircoTYPErs (VircoTYPE is the new name for VirtualPhenotype) have to revamp their reports to weigh the import of K20M, L33F, and I47V, for example, versus L10V, K20R, and I54A? Will clinicians have to memorize this sesquipedalian substitution chart, or at least keep it at their fingertips?
Probably not. Boehringer has already expended much effort reckoning simpler ways to gauge tipranavir’s potential in people with PI-resistant virus. First, one can measure the fold-change in susceptibility to tipranavir before starting the drug. Anything below 1 is splendid. A fold-change below 4 foretells more than a half-log drop in viral load after 24 weeks in most people. But a baseline fold change above 4 means many people won’t respond (Table 2.2).
Table 2.2. Response to tipranavir based on baseline susceptibility to the PI
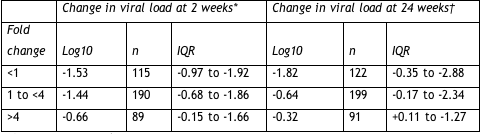
*On-treatment analysis.
†Last-observation-carried-forward analysis.
IQR = interquartile range.
This analysis is not cut-and-dried, Mayers noted, because most people with low susceptibility to tipranavir at baseline (more than a 4-fold change) had few active drugs to add to their new tipranavir regimen. So these people got less help from tipranavir than others–and little help from other antiretrovirals. Even so, the interquartile range shows that 25% of this group garnered more than a 1.27-log drop in viral load after 24 weeks.
A multiple regression model of virologic response to tipranavir salvage at 24 weeks picked out four independently predictive factors: use of tipranavir itself, use of enfuvirtide (T-20), more active drugs in the background regimen, and, yes, the tipranavir resistance score (P < 0.01 for all).
The 21-mutation tipranavir score did much better than the IAS-USA list of primary protease mutations in foretelling the response to tipranavir at 2 weeks. People whose virus carried up to seven of the 21 critical mutations had more than a 1-log drop in viral load at week 2, whereas people with eight of the 21 mutations did not respond. A score that included all IAS-USA protease mutations saw no difference in 2-week response until virus bore 13 mutations.
So at this point Boehringer predicts that most people with more than seven mutations from the set of 21 will respond poorly to tipranavir. But company virologists have not entirely tossed out the four-mutation set of changes at positions 33, 82, 84, and 90, arguing that they denote a highly resistant virus that tipranavir will struggle to control. But many may find it odd to juggle two scores–one that includes L90M and one that does not.
References
(To view slides and posters from the Third European HIV Drug Resistance Workshop, go to http://www.hivpresentation.com.)
1. Cooper D, Hicks C, Cahn P, et al. 24-week RESIST study analyses: the efficacy of tipranavir/ritonavir is superior to lopinavir/ritonavir, and the TPV/r treatment response is enhanced by inclusion of genotypically active antiretrovirals in the optimized background regimen. 12th Conference on Retroviruses and Opportunistic Infections. February 22-25, 2005. Boston. Abstract 560.
2. Schapiro J, Cahn P, Trottier B, et al. Effect of baseline genotype on response to tipranavir/ritonavir compared with standard-of-care comparator in treatment-experienced patients: the phase 3 RESIST-1 and -2 trials. 12th Conference on Retroviruses and Opportunistic Infections. February 22-25, 2005. Boston. Abstract 105.
3. Leith J, Walmsley S, Katlama C, et al. Pharmacokinetics and safety of tipranavir/ritonavir alone or in combination with saquinavir, amprenavir, or lopinavir: interim analysis of BI1182.51. 5th International Workshop on Clinical Pharmacology of HIV Therapy. April 1-3, 2004. Rome. Abstract 34.
4. Larder BA, Hertogs K, Bloor S, et al. Tipranavir inhibits broadly protease inhibitor-resistant HIV-1 clinical samples. AIDS 2000;14:1943-1948.