| |
Epidemiology, Natural History, Impact of Therapy of Hepatitis B
|
| |
| |
- Epidemiology of Hepatitis B
- Candidates for Screening
- Diagnostic Tests for Screening and Evaluation in Hepatitis B
- Further Evaluation for HBsAG+ Patients
- Warning Signs of Serious Liver Disease
- Vaccination
- natural history
- chronic HBV
- impact of therapy on natural history
.....Hepatitis B virus (HBV) is transmitted via parenteral or mucosal exposure to body fluids (semen) of individuals who are acutely infected with or are chronic carriers of HBV. The highest viral concentrations are found in the blood and serous fluids....
......Chronic infection has been observed to evolve in up to 90% of infected infants of HBeAg positive mothers, in about 30% of children infected at <6 years of age, and probably in <5% of infected older children and adults....
www.projectsinknowledge.com/HBV
Robert G. Gish, md
Medical Director
Liver Transplant Program
Chief, Division of Hepatology and Complex GI
California Pacific Medical Center
San Francisco, California
Conclusions
Hepatitis B is a common and potentially serious infection. Worldwide, there are more than 4 million acute cases each year, and approximately 350 million people are chronic carriers.1 In areas of high endemicity- southeast Asia and the Pacific Basin (except for Japan, Australia, and New Zealand), sub-Saharan Africa, the Amazon Basin, parts of the Middle East, the central Asian republics, and some eastern European countries-up
to 90% of the population becomes infected with hepatitis B virus (HBV) by age 40 years, 8% to 20% of whom become carriers.1 Areas of low endemicity, where infection rates are <20% and carrier rates are <2%, include North America, western and eastern Europe, Australia, and parts of South America.1
Hepatitis B is a common and potentially serious infection. Worldwide, there are more than 4 million acute cases each year, and approximately 350 million people are chronic carriers.1 In areas of high endemicity— southeast Asia and the Pacific Basin (except for Japan, Australia, and New Zealand), sub-Saharan Africa, the Amazon Basin, parts of the Middle East, the central Asian republics,
and some eastern European countries—up to 90% of the population becomes infected with hepatitis B virus (HBV) by age 40 years, 8% to 20% of whom become carriers.1 Areas of low endemicity, where infection rates are <20% and
carrier rates are <2%, include North America, western and eastern Europe, Australia, and parts of South America.1
In the United States, reported cases of acute hepatitis B peaked at 26,000 per year in th mid-1980s and have since declined steadily to about 7526 reported cases in 2003.2-4 This decline is largely attributable to HIV prevention efforts that reduced transmission among homosexual men and injection drug users, and to vaccination of children.2,3 The Centers for Disease Control and Prevention (CDC) estimates that in the early 2000s, there have been about 73,000 to 80,000 new infections each year, which have led to about 21,000 to 23,000 new acute clinical infections annually (only about 7500–8000 of which are reported),4 and that there are 5000 to 8000 new chronic infections each year.2 Currently, 1 to 1.25 million persons in the United States are chronically infected.2,4 Thus, identification and treatment of hepatitis B remain important concerns, even in areas of "low" endemicity, like the United States.
HBV is transmitted through parenteral or mucosal exposure to body fluids of carriers or acutely infected individuals.2 In areas of high endemicity, hepatitis B is most commonly transmitted perinatally from the mother or through infection in early childhood. Due to their more immature immune systems,
children are much more likely to develop chronic infection than adults.5 Since mother to- child transmission accounts for the majority of hepatitis B cases in areas of high endemicity, these areas have higher rates of chronicity than
low-endemicity areas.5 Moreover, the higher prevalence of chronic infection also translates into higher rates of cirrhosis and cancer in these areas. In the United States, hepatitis B is mostly an adult-acquired disease associated with high-risk behavior, including sexual contact and injection drug use.2 However, it is important to consider that the United States is a country of immigrants, many of whom arrive from areas of high endemicity. For example, although the prevalence of HBV infection is only 0.3% in the general US population, it is as high as 15% among Asians and Pacific Islanders, who comprise 1.3 to 1.5 million known carriers of hepatitis B in the United States.6
The need for concern about hepatitis B stems not only from its prevalence but also from its potentially serious sequelae. Persons with chronic HBV infection are at risk for cirrhosis and hepatocellular carcinoma (HCC), which occur in about 20% of cases (annual incidence: 5.9%).7 At 5 years after diagnosis of compensated cirrhosis, the cumulative probability of HCC is 6% and that of
decompensation is 23%.8 It should also be noted that hepatitis B is a systemic disease and can, in rare cases, result in nonhepatic end-organ injury, including vasculitis, kidney failure, pancreatitis, nerve damage, and other forms of tissue injury.
Candidates for Screening
Screening for hepatitis B is routinely performed in persons who have elevated liver enzyme levels, jaundice, or other evidence of acute liver disease. In addition, screening is warranted for anyone with risk factors for HBV infection, and at-risk persons who are found to be susceptible to hepatitis B should receive vaccination against HBV.
At-risk individuals include 2,9:
- Those who have engaged in high-risk sexual behaviors (men who have sex with men, anyone with multiple heterosexual sex partners, and persons recently diagnosed with a sexually transmitted disease)
- Injection drug users
- Immigrants, refugees, or adoptees/orphans from areas of high endemicity
- Those who are immunosuppressed (eg, transplant recipients, those on
immunosuppressive therapies, those with autoimmune diseases or HIV infection, and patients receiving chemotherapy)
- Dialysis patients
- Household members or sexual partners of known HBV carriers
- Those who have had occupational exposure through needle sticks
- Recipients of certain blood products (eg, clotting factors for hemophilia)
- Inmates in long-term correctional facilities or residents in an institution for the
developmentally disabled
The risk of hepatitis B through blood transfusion varies throughout the world.
In countries with similar blood-screening systems—the United States, Europe, and Australia—the risk for HBV can range from 1 in 63,000 blood units to 1 in 398,499.10,11
Diagnostic Tests for Screening and Evaluation in Hepatitis B
An initial screen for hepatitis B should consist of hepatitis B surface antigen (HBsAg), hepatitis B core antibody (anti-HBc), and hepatitis B surface antibody (anti-HBs). HBsAg positivity is a general marker of acute or chronic infection. It is the first marker......
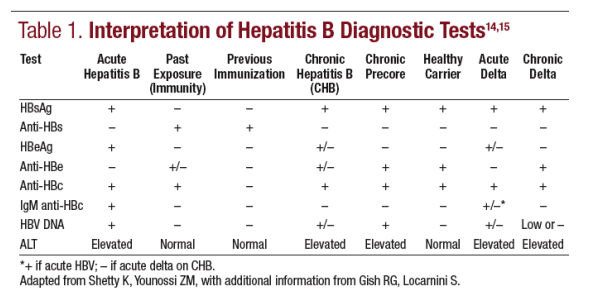
to appear, and its persistence for >6 months indicates chronic infection. Anti-HBc is a marker of exposure that persists for life.
Anti-HBs documents recovery and/or immunity to HBV and is detectable
after immunity conferred by hepatitis B vaccination or previous infection. There is some controversy regarding the need for, and the cost-effectiveness of inclusion of, all three tests in the initial screen. Some healthcare authorities have advocated that only the HBsAg test, or only the anti-HBc test, be used;
however, the full panel appears to be the most effective means of screening an individual for the presence of infection, exposure, or immunity.
Further Evaluation for HBsAg+ Patients
Individuals with HBsAg positivity should undergo further workup that includes hepatitis B e antigen (HBeAg), hepatitis B e antibody (anti-HBe), and HBV DNA quantification by nucleic acid testing.
An optional test is the anti-HBc IgM, which is the best test to document an acute infection; if positive in a person known to have chronic infection, it imparts a worse prognosis.12,13 Another optional test in patients with adult-acquired HBV
infection is a test for hepatitis delta antibody. Interpretation of these tests is described in Table 1.14,15 Liver biopsy is useful in deciding which patients with
chronic infection to treat and when to initiate therapy.
Precore mutant chronic hepatitis B is found in about 10% to 40% of cases in northern Europe and the United States and 30% to 80% in southern Europe and Asia.16 Patients with precore mutant chronic hepatitis B are HBsAg+, are HBeAg– , generally have HBV DNA levels 30,000 to >100,000 copies/mL, and typically have elevated alanine aminotransferase (ALT) levels. This variant of chronic hepatitis B is suspected, but not proven, to be associated with a high
risk of cirrhosis,16 and it is more difficult to treat,17 though response may be better with peginterferon than with nucleoside analogs like lamivudine.18
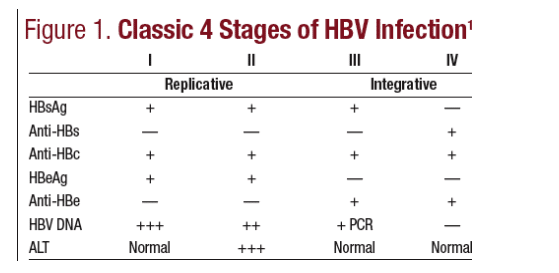
Serologic findings also vary over time with the stage of infection (See Figure 1).19 In the first stage of viral replication, patients generally are asymptomatic and have normal ALT levels. In the second phase of replication, an immunologic response develops or increases and ALT levels tend to become elevated as
HBV DNA levels decline. Replication ends as the virus is cleared, but this is followed by a phase of integration of HBV into the hepatocyte genome. This is a quiescent phase with low HBV DNA levels and normal or slightly abnormal ALT levels. The final phase is clearance of HBsAg and HBV DNA, which can occur naturally or through therapeutic intervention. HBV is not considered a curable disease, because even when viral replication is stopped, the virus persists in an inactive form. Persons who are HBsAg– may still have tiny amounts of HBV DNA in their liver tissue. However, though "not cured," most patients with previous exposure who are not chronically infected and lack HBsAg have minimal longterm risk from HBV.
Additional tests are currently available to some practitioners in the United States,
including tests for hepatitis B genotype, and direct tests for precore and core promoter mutations. It has been proposed that these tests may provide further information regarding prognosis, likelihood of treatment response, risk of developing treatment resistance, and risk of cancer.
Nucleic Acid Testing
As noted above, every patient who is HBsAg+ should have HBV DNA
testing. In acute HBV infection, a high HBV DNA level indicates a low
probability of clearance, whereas clearance is more likely among those with
low HBV DNA levels.
In chronic infections, serum HBV DNA level has been found to correlate with histologic grading as well as biochemical and serologic response to treatment.20,21 The test should be repeated every 6 months for those not on
therapy in order to identify any changes in replication status. In addition, for those on therapy, HBV DNA testing is used every 3 to 6 months, or immediately after stopping therapy, to monitor treatment response. It can be useful in evaluating the rare patient who is HBsAg– but has active viral replication.
A number of nucleic acid tests measure HBV DNA, including polymerase chain
reaction (PCR), branched-chain (bDNA) assay, transcription-mediated amplification (TMA), hybrid capture, and column hybridization.
The latter two are of historical value but are now rarely used.
The PCR assay copies the viral DNA to another DNA molecule, and is the standard test used for almost all licensing trials since it denotes the most profound level of viral suppression. The test with the lowest range of detection is the COBAS Amplicor HBV Monitor PCR assay, which can detect as few as 200 copies/mL. Taqman, a new PCR test currently in development, is anticipated
to have a higher level of sensitivity and the widest dynamic range, but it is not yet fully developed for broad-based clinical use.
The bDNA assay has a dynamic range of 2000 to 100 million copies/mL. Dilution extends its range to 5 billion copies/mL. This test was used in some licensing studies, including studies of emtricitabine and entecavir.
A new qualitative PCR test, the COBAS AmpliScreenTM, can detect as few as
15 copies/mL, and the Procleix UltrioTM TMA assay can detect down to 25 copies/mL. In the future, these qualitative tests may be able to be converted to quantitative tests.
Warning Signs of Serious Liver Disease
Hepatitis B potentially has serious clinical consequences including cirrhosis, liver failure, and HCC.2 In the United States, HBV-related cirrhosis kills about 3000 to 4000 persons each year, and another 1000 to 1500 die of HBV-related liver cancer.2
It is important to recognize the warning signs of advanced liver disease. In acute liver disease, ALT > 500 IU/L, encephalopathy, and jaundice are signs of serious liver disease. The presence of encephalopathy indicates fulminant hepatic failure. In chronic infections, warning signs of serious liver disease include prolonged prothrombin time or institutional normalized ratio (INR), bilirubin > 2 mg/dL, albumin < 3.5 g/dL, cholesterol < 100 mg/dL, ammonia > 65 mmol/L, and platelets < 100,000 mm3.
Signs/symptoms of serious liver disease found on physical examination include swollen feet or abdomen, confusion, progressive memory loss, sleep difficulties,
vomiting blood/blood in the stool, bleeding/hemorrhages (including gastrointestinal bleeding and skin bleeding), jaundice, flapping of hands when arms are extended, and muscle wasting. Such patients should be referred to specialists who manage advanced liver disease or to liver transplant centers.
Vaccination
The only way to eliminate hepatitis B globally is through vaccination. All infants, children, and adolescents should now be vaccinated routinely.2 All pregnant women should be screened, and vaccination and immunoprophylaxis provided to their infants if appropriate.2 All susceptible adults with any of the risk factors described earlier in this newsletter should be vaccinated.2
An anti-HBs level > 10 mIU/mL indicates vaccine immune response, which is achieved in 95% of children and 90% of adults.2
Factors associated with reduced immune response to vaccine include older age (~90% seroprotection for persons >40 years and ~75% for persons >60 years),2 chronic medical disease, buttock injection site, smoking, obesity, and male gender.2 Postvaccination serologic testing is not routinely performed, but there are some exceptions.
Serologic testing at 1 to 2 months following completion of vaccination is recommended if knowledge of immune status would affect management,2 as might be the case, for example, for dialysis patients, immunocompromised
persons at continued risk, sexual partners of patients with chronic HBV infection, and people with occupational risk of blood exposure.
Postvaccination testing at 9 to 15 months of age is also recommended for infants born to HBsAg+ mothers.2 Nonresponders (ie, anti-HBs < 10 mIU/mL) at high risk for infection should be revaccinated by repeating a three-dose vaccine series, which produces response in 30% to 50% of adults.2
NATURAL HISTORY: From Exposure to Acute Infection
Hepatitis B virus (HBV) is transmitted via parenteral or mucosal exposure to
body fluids of individuals who are acutely infected with or are chronic carriers of
HBV. The highest viral concentrations are found in the blood and serous fluids.1
In the Western World, HBV infection is most commonly acquired in adulthood
via percutaneous exposure or sexual contact. In contrast, in endemic areas
such as sub-Saharan Africa and the Far East, HBV infection is most commonly
acquired from an infected mother at birth or during early childhood.
Initial exposure to the virus is followed by an incubation period of 6 weeks to
6 months.1 Most children and about half of all adults who have acute infections
are asymptomatic. A preicteric/prodromal phase lasts approximately 3 to 10 days, from initial symptoms to onset of jaundice. Symptoms of acute infection
are nonspecific and include nausea/ vomiting, anorexia, fatigue, malaise, pain
in the right upper abdominal quadrant, fever, headache, myalgia, arthralgia,
arthritis, skin rash, and dark urine. The preicteric phase is succeeded by the icteric phase, lasting 1 to 3 weeks. During this phase, patients may have jaundice, light or gray stools, hepatic tenderness, and hepatomegaly. Malaise and fatigue may last for weeks or months during convalescence, while other symptoms disappear. Fulminant hepatitis develops in 1% to 2% of cases and is associated with a mortality rate of 63% to 93%.1
Children who are acutely infected are more likely to develop chronic disease than those who acquired HBV in adulthood. Chronic infection has been observed to evolve in up to 90% of infected infants of HBeAg positive mothers, in about 30% of children infected at <6 years of age, and probably in <5% of infected older children and adults.2 Viral clearance in the acute phase is most common among those who mount a vigorous immune response, as characterized by elevated ALT levels and jaundice, and this is more likely among those who are exposed to HBV in adulthood.
Chronic HBV Infection
When the virus is not cleared in the acute phase and continues to replicate, there is a potential for clinical disease. Persistence of HBsAg for >6 months, often high serum HBV DNA > 105 copies/mL (100,000), persistent or intermittent elevations in ALT/AST levels, and liver biopsy showing chronic hepatitis indicate chronic infection.
Prognosis of Chronic Hepatitis B
Over time, a minority of patients with chronic infection spontaneously clears the virus. Although most patients lose HBeAg (up to 80% at 10 years), few (about 5%) lose HBsAg.6 Patients who remain HBsAg positive are still at risk for reactivation of viral replication.
Without treatment, chronic active infection can be progressive. About 30% of those with chronic HBV infection progress to cirrhosis.2 Within 5 years, cirrhosis progresses to liver failure in about 23% and to HCC in about 6%.7 About 5% to 10% of chronically infected individuals develop HCC in the absence of cirrhosis.2 Risk factors for HCC among those with chronic infection include cirrhosis, residence in an area of high endemicity, older age, male gender, sustained activity of liver disease, coinfection with hepatitis C virus, HBeAg positivity, high HBV DNA levels, alcohol abuse, family history of liver cancer, smoking, and aflatoxin exposure.8-10 Some patients also experience an acute flare-up of
chronic infection, which can mimic acute hepatitis. This phenomenon can have serious consequences, particularly for those with cirrhosis. These flares can be spontaneous, can result from treatment interruption, can occur with the use of immunosuppressant drugs for any reason, or can occur following the development of treatment-resistant viral mutations.
Five-year survival is 55% to 84% for those with compensated cirrhosis and 14% for those with decompensated cirrhosis.11,12 Risk factors for mortality related to HBV infection include HBeAg positivity, older age, race other than white or black, male gender, bilirubin ≥ 1.5 mg/dL, ascites, and the presence of spider nevi.11,12
Impact of Treatment on Natural History
Although direct evidence from long-term prospective trials is lacking (recently at hepatitis conferences--DDW, EASL 2005—study data has been presented showing HBV DNA levels were associated with improved outcomes), indirect evidence supports the belief that treating chronic hepatitis B would favorably impact on the natural history and improve survival. Viral replication/HBV viremia is associated with progressive disease; therefore, it is logical that suppression of viral replication and loss of HBeAg would improve long-term outcomes.
Treatment, however, would have to consider several factors apart from viral
replication, and these include the natural history of liver disease in the various clinical profiles, the probability of response to therapy in a given situation, the histologic severity of liver disease, and the cost of the therapy, as these are likely
to influence the risk/benefit ratio. High levels of HBV DNA have been
shown in longitudinal studies to correlate with histologic activity, progression of disease to cirrhosis, increased risk of HCC, and mortality from liver
disease.13-19 In contrast, inactive carriers with undetectable HBV DNA, normal
ALT levels, and loss of HBeAg rarely show signs of progression or histologic abnormality, as mentioned above.
Histologic improvement has also been documented in studies of antiviral therapy
for chronic hepatitis B. For example, studies have shown histologic improvement in 53% to 64% of patients treated with adefovir.20,21 In randomized studies, histologic improvement was observed in 70% to 72% of those treated with entecavir and 61% to 62% of those treated with lamivudine.22,23 In recent studies, histologic improvement was reported in 41% to 48% of patients treated with peginterferon alfa-2a and 40% to 41% of those treated with lamivudine.24
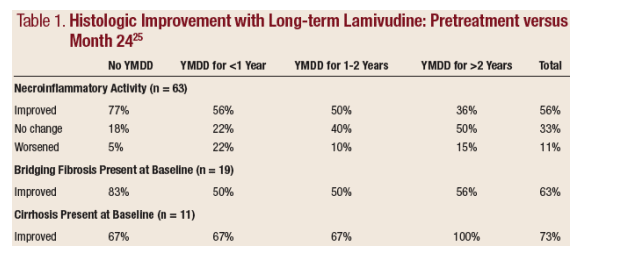
Dienstag et al25 also provided important evidence of histologic improvement with
treatment in an evaluation of patients on longterm lamivudine. In this study, 63 patients underwent three sets of liver biopsies: one pretreatment, one after 1 year of lamivudine therapy, and another after an additional 2 years of lamivudine. After 3 years of treatment, necroinflammatory activity was reduced in 56% of patients. Another third showed no change, and only 11% showed worsening. Those who developed YMDD variants, which is associated with treatment
resistance, were least likely to improve.
Importantly, bridging fibrosis improved by ≥1 level in 63% and cirrhosis improved in 73%. Only 2% progressed to cirrhosis and 9% (all with YMDD variants) progressed to bridging fibrosis (See Table 1). This important study provides some of the best available evidence that treatment does affect natural
history and can even reverse fibrosis and cirrhosis in many patients.
In addition to histologic improvement, treatment has also been shown to improve
clinical outcomes. For example, in 23 patients with severely decompensated HBV-related cirrhosis, lamivudine treatment was associated with clinical response (ie, ≥3-point decrease in Child-Turcotte-Pugh score) in 60.9% versus
none of 23 historical controls (P < .0001).
Lamivudine-treated patients also had significantly fewer transplants, experienced
longer time to transplantation, and exhibited a survival advantage.26 One could assume that similar results will be seen with effective therapy with other agents. One may also speculate that with the lower incidence of resistance with adefovir and entecavir, the improvement histologically and clinically may be even better, but time will tell.
Treatment needs to be tailored for different subgroups of patients in order to maximize its effect on long-term outcomes. Patients with wild-type HBV (HBeAg positive patients) can have a durable response to therapy of finite duration with suppression of HBV DNA and HBeAg seroconversion. In contrast, those with precore mutant virus have a high rate of relapse after treatment discontinuation and therefore require indefinite therapy to maximize treatment benefits. The optimal duration of therapy in patients with cirrhosis is controversial, but Dr. Reddy believes that treatment should be indefinite for this population as well, since reactivation can lead to worsening liver function and decompensation.
Case Study
A 46-year-old man presented with complaints of fatigue. Physical examination showed him to be anicteric with occasional spider angiomata, a liver span of 16 cm, and no splenomegaly. Laboratory analysis showed ALT 78 IU/L, AST 89 IU/L, alkaline phosphatase 133 IU/L, albumin 3.6 g/dL, total bilirubin 1.2 mg/dL, INR 1.1, and platelets 138,000/_L. His viral hepatitis tests indicated that he was positive for HBsAg and HBeAg, and negative for anti-HBe, anti-HCV [hepatitis C virus], and anti-delta.
His HBV DNA level was 85,000 copies/mL. Abdominal ultrasound showed no masses in the liver and no ascites. He had no evidence of encephalopathy or variceal bleeding. Although there was no evidence of cirrhosis, the patient was worried about a "missed diagnosis" and requested a liver biopsy, which showed histologic evidence of cirrhosis. The patient’s physician recommended treatment with a nucleoside or nucleotide analog, but he was reluctant due to concerns
regarding side effects and resistance rates with oral agents. The patient returned in 6 months with mild ascites and requested to be considered for HBV therapy to prevent further progression and liver transplantation.
Workup showed no evidence of HCC. At that time he was positive for HBeAg and
anti-HBe, and had an HBV DNA level of 94,000 copies/mL. His model for end-stage liver disease (MELD) score was 9. The patient refused lamivudine due to the risk of developing early resistance. His physician prescribed adefovir, and his ascites was well controlled with spironolactone. In a situation such as this, entecavir, the more recently approved nucleoside analog, would be another option.
Four months later, the patient felt well. His MELD score decreased to 7, and he was HBV DNA negative by polymerase chain reaction; however, he remained HBeAg positive. One year later, he continues to feel well and is negative for both HBV DNA and HBeAg. He is anti-HBe positive and HBsAg positive. He has so far avoided the need for liver transplantation. His physician recommended that he continue therapy indefinitely to reduce the long-term risk of decompensation and HCC.
Conclusion
As the case study illustrates, untreated chronic HBV infection can be progressive
with serious clinical consequences. However, treatment has the potential to alter the natural progression of the disease and, in some cases, reverse even advanced fibrosis and cirrhosis.
|
|
| |
| |
|
|
|