| |
High-dose ribavirin in combination with standard dose peginterferon for treatment of patients with chronic hepatitis C
|
| |
| |
Hepatology
Volume 41, Issue 2, February 2005
SEE EDITORIAL BELOW
Karin Lindahl 1 *, Lars Stahle 2, Annette Bruchfeld 3, Robert Schvarcz 1§
1Departments of Infectious Diseases, Karolinska University Hospital Huddinge, Karolinska Institute, Stockholm, Sweden
2Clinical Pharmacology, Karolinska University Hospital Huddinge, Karolinska Institute, Stockholm, Sweden
3Renal Medicine, Karolinska University Hospital Huddinge, Karolinska Institute, Stockholm, Sweden
ABSTRACT
Improved treatment regimens for patients with chronic hepatitis C, genotype 1 and high viral load are needed. Increasing the dose of ribavirin has increased the response rate, but experience with doses of more than 1,200 mg/day is limited. The aim of this study was to investigate the safety and tolerance to treatment with a high and individualized dose of ribavirin in combination with peginterferon. Ten patients with chronic hepatitis C, genotype 1 and high viral load were treated with peginterferon alfa-2a and ribavirin for 48 weeks in a prospective trial. The initial ribavirin dose was individualized and calculated from a pharmacokinetic formula based mainly on renal function. Ribavirin plasma concentrations were monitored, and the dose was adjusted to reach the target concentration. Hemoglobin was monitored, and patients were treated with erythropoietin and blood transfusions when indicated. After dose adjustments, the mean dose of ribavirin was 2,540 mg/day (range, 1,600-3,600) at week 24. The main side effect was anemia, which was controlled with erythropoietin. [note from Jules: mean hemoglobin was 9.7 at week 24. All patients received erythropoietin]. Two patients required blood transfusions. One patient was withdrawn at week 24 because of a lack of viral response, and one patient at week 39 because of side effects, primarily interferon associated. At follow-up (24 weeks posttreatment), nine of ten patients had undetectable HCV RNA and thus were cured by standard definitions. In conclusion, a high dose of ribavirin according to an individualized schedule is feasible but associated with more frequent and serious side effects such as anemia. The viral response merits further evaluation.
Viral Response.
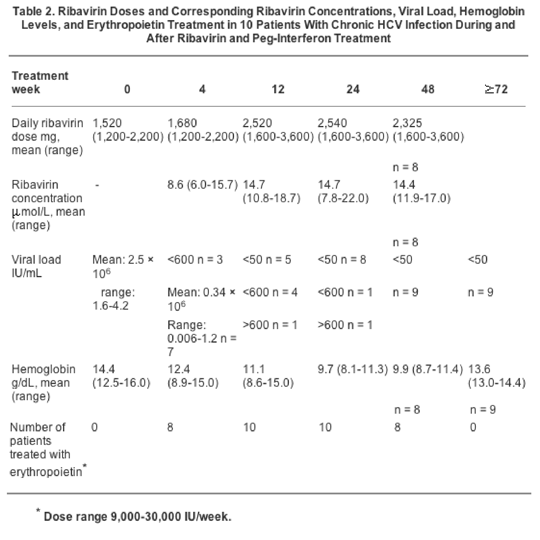
Table 2 shows the viral response. In one patient, the treatment was discontinued after 24 weeks because of HCV RNA levels >600 IU/mL. The remaining nine patients became HCV RNA negative during treatment and remained negative at follow-up 24 weeks after the cessation of treatment, including one patient who was HCV RNA positive (<600 IU/mL) at week 24 and one patient who discontinued the treatment at week 39 because of side effects.
Side Effects.
Hemoglobin levels decreased during the treatment, as shown in Table 2 (see tables below). In two patients, the hemoglobin levels decreased to below 8.0 g/dL (nadir, 7.3 and 7.9, respectively). These patients received blood transfusions on two occasions each, and the dose of ribavirin was reduced or temporarily discontinued for 1 week. An additional 5 patients experienced a decrease in hemoglobin to levels between 8.0 and 10.0 g/dL, and two of these patients had minor dose reductions of ribavirin. Mean hemoglobin was 9.7 (8.1-11.3) at week 24 & similar at week 48. At week 72 mean Hg was 13.6 (13.0-14.4). For shorter periods, in particular during the first 3 to 6 months of treatment, the hemoglobin levels were measured once per week. Nausea caused a minor dose reduction in one additional patient. During the course of treatment, all patients received erythropoietin with doses in the range of 9,000 to 30,000 IU/week as well as an oral iron supplement.
One patient discontinued ribavirin at week 39 because of side effects, mainly associated with concurrent interferon treatment. In four patients peginterferonalfa-2a was discontinued for a short period or a reduced dose was given because of neutropenia.
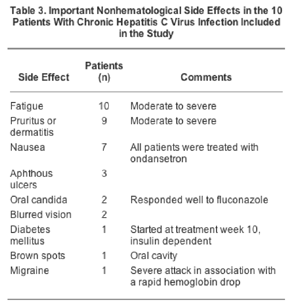
Important nonhematological side effects are shown in Table 3. Fatigue, nausea, and dermatological problems were, in our opinion, more frequent and severe problems than those usually observed with standard combination treatment. The working capacity was reduced in all patients. One patient, a former drug addict, relapsed into sporadic intravenous amphetamine use 1 month after completing the treatment and was initially lost to follow-up, but came for his follow-up visit 10 months after cessation of treatment. The mean weight (range) in kilograms, at baseline, treatment week 12, and treatment week 20, was 80.6 (66.5-109.0), 79.0 (63.5-110.0), and 78.0 (62.9-110.0), respectively.
EDITORIAL
Pushing the treatment envelope for chronic hepatitis C - is more necessarily better?
Glen Lutchman, M.D., Marc Ghany, M.D. *§
Liver Diseases Branch National Institute of Diabetes and Digestive and Kidney Diseases National Institutes of Health Bethesda, MD
Article Text
Ribavirin is a synthetic purine analogue that has a broad spectrum of antiviral activity and is approved for use in combination with interferon for the treatment of chronic hepatitis C. Clinically, it appears to act synergistically with interferon to result in a small increase in end-of-treatment response but more importantly a doubling of the sustained virological response (SVR) rate by preventing virological relapse. In vitro, ribavirin has been shown to act as an immunomodulator, a competitive inhibitor of inosine monophosphate dehydrogenase, a direct inhibitor of the viral RNA dependent RNA polymerase and as a viral mutagen. However, it is unclear which, if any, of these mechanisms is important for its antiviral effect in vivo.
The recommended therapy for patients with chronic hepatitis C is the combination of either peginterferon alfa-2a or -2b once weekly plus daily ribavirin for 48 weeks.[1][2] The recommended dose of ribavirin is dependent on the preparation of peginterferon used. In the United States, ribavirin is approved for use with peginterferon alfa-2b at a fixed dose of 800 mg daily, but in combination with peginterferon alfa-2a, ribavirin is given in a dose of 1,000 or 1,200 mg based on whether body weight is less than or greater than 75 kg, respectively. In Europe, the approved daily dose is based on weight regardless of peginterferon preparation: 800 mg for patients below 65 kg; 1,000 mg for those between 65 and 85 kg; and 1,200 mg for patients heavier than 85 kg. The decision in the United States. to approve a lower, fixed dose of ribavirin in combination with peginterferon alfa-2b was based on Food and Drug Administration concerns about the lack of data on efficacy and more importantly, the safety of higher doses of ribavirin.[3] However, in a secondary analysis of the peginterferon alfa-2b plus ribavirin registration trial, body weight was identified as an important predictor of response and the European regulatory agency chose to approve weight-based ribavirin.[1] The approved regimens yield similar SVR rates ranging from 54% to 63%.[1][2][4] In an effort to improve SVR rates, investigators have experimented with different regimens by varying the dose and duration of therapy. A theme emerging from recent studies is that therapy should be tailored to different groups of patients. The current data suggest that patients with genotype 1 should be treated for 48 weeks, whereas those infected with genotypes 2 and 3 might be treated for a shorter duration of 24 weeks and perhaps as short as 14 to 16 weeks in patients who achieve an early virological response (hepatitis C virus RNA negative at treatment week 4).[5]
A controversial issue in chronic hepatitis C therapy is the optimal dose of ribavirin to use in combination with peginterferon and whether higher doses of ribavirin are more effective. The initial evidence supporting higher doses of ribavirin was largely indirect and evolved from a secondary analysis of the large multicenter trial of peginterferon alfa-2b and ribavirin. A logistic regression analysis revealed that, if the ribavirin dose was expressed as mg/kg, patients who received higher doses experienced the highest SVR rates.[1] Using an arbitrary ribavirin dose cutoff of 10.6 mg/kg, genotype 1 patients who received the standard dose of peginterferon alfa-2b (1.5 g/kg/wk) and higher doses of ribavirin (>10.6 mg/kg) were shown to have higher SVR rates compared with those receiving lower doses (<10.6 mg/kg), 48% versus 38%. Although suggestive that higher doses of ribavirin were better, these data should be interpreted with caution because they were derived from a secondary analysis with the potential for confounding variables and the small numbers of patients within subgroups. More compelling data came from a recent randomized trial of peginterferon alfa-2a and ribavirin which was specifically designed to assess efficacy of low-dose versus standard dose ribavirin. The results clearly showed that for genotype 1 patients treated for 48 weeks the SVR rate was superior in those receiving standard dose (1,000 to 1,200 mg) compared with those receiving low-dose ribavirin (800 mg), 52% versus 41%.[4] In contrast, both studies showed no difference in the SVR rate in patients with genotypes 2 and 3 infections using ribavirin doses ranging from 800 to 1,200 mg daily. Thus, the issue can be narrowed to optimizing therapy for genotype 1 patients.
In this issue of HEPATOLOGY, Lindahl and coworkers provide some further data regarding the benefit of higher doses of ribavirin for genotype 1 patients.[6] They conducted a small pilot study in which they treated 10 patients with standard doses of peginterferon alfa-2a and individualized the daily dose of ribavirin to achieve a target ribavirin concentration in serum of 15 mol/mL. The primary goal was to evaluate the safety of using higher doses of ribavirin in patients with genotype 1 and high viral load (the difficult-to-treat patient profile). To achieve the targeted serum concentration, patients required a mean ribavirin dose of 2,540 mg/day (range 1,600-3,600 mg/day), which is more than twice the currently recommend maximum daily dose. However, in an intention-to-treat analysis of 10 patients, the SVR rate was an impressive 90%. Side effects were severe, particularly hemolysis and anemia. Every patient required erythropoietin, and two patients required blood transfusion on two separate occasions.
The results of this study are indeed striking but a note of caution must be struck. Foremost is the issue of safety. One of the major limitations to combination therapy is the high frequency of side effects, some of which may be serious and life-threatening. In the two large multicenter registration trials, 10% to 14% of patients required discontinuation of therapy and 32% to 42% required dose modification for serious or severe side effects.[1][2] Higher doses of ribavirin would undoubtedly lead to more side effects. Hemolytic anemia is the major risk associated with ribavirin, and, if defined as a hemoglobin level less than 10g/ dL, occurred in 7 of 10 patients. All patients required hemopoetic growth factor, two required blood transfusion and four required dose reduction or temporary discontinuation of the drug to manage side effects. Furthermore, all patients experienced a reduction in ability to work, although, quality-of-life was not specifically assessed. The need for erythropoietin, blood transfusion, and loss of work are not trivial matters particularly in the typical patient with hepatitis C who has few if any symptoms and is largely fully functional. Anemia caused by ribavirin can induce cardiovascular and cerebrovascular incidents in susceptible patients. Thus, even with intensive monitoring this high-dose ribavirin regimen can be life-threatening. Second, we should recognize that the study by Lindahl et al. was a small, nonrandomized pilot one without a control group. Finally, difficult-to-treat patients such as African Americans, those with significant comorbidities and patients with cirrhosis, all of whom have been shown to have lower SVR rates, were not included.[7][8]
Another concern in today's practice environment is the cost associated with this treatment regimen. A 48-week course of standard treatment without the need for supplementary therapy or testing costs approximately 24,000 U.S. dollars.[9] The additional cost associated with a high-dose ribavirin regimen would not be insignificant and include paying for a greater quantity of ribavirin, the need to monitor ribavirin levels, more frequent laboratory monitoring, use of erythropoietin, blood transfusion, additional clinic visits, hospitalizations, and time missed from work. The obvious question is whether the additional risks and costs are worth the increase in SVR. For genotype 2 and 3 patients who already have high SVR rates with standard peginterferon and low-dose ribavirin, the incremental benefit of higher ribavirin doses is likely to be minimal and not worth the added risks and costs. Similarly, it would be difficult to justify this high-dose ribavirin regimen for treatment naïve genotype 1 patients given a SVR rate of 42% to 47% with standard therapy. Such an approach would unnecessarily expose close to 50% of genotype 1 patients to a potentially more toxic therapy. For the moment, we would suggest that until more data and experience are available, this regimen should not be offered outside the setting of a clinical trial. Ideally, this approach should be evaluated in a prospective controlled trial using patients who have failed to respond to standard therapy.
The finding of Lindahl and colleagues that higher doses of ribavirin may improve the SVR rate suggests that a safer ribavirin preparation is required and that ribavirin-like compounds with better safety profiles may have a role to play in therapy. Viramidine, a prodrug of ribavirin that specifically targets the liver, is associated with less hemolysis than ribavirin, and in preliminary clinical studies, seems to have similar efficacy.[10] Levovirin, the L-enantiomer of ribavirin, has been disappointing thus far in clinical trials but higher doses have not been tested.[11]
The apparent increase in effectiveness of high-dose ribavirin over standard dose is intriguing from a mechanistic view. At higher doses, ribavirin may either augment previously proposed antiviral pathways, act through a novel mechanism or, perhaps, at higher doses more patients achieve a critical threshold of intracellular ribavirin concentration. Unfortunately, the results of Lindahl and coworkers seem to raise more questions than to provide answers as to the mechanism of action of ribavirin. Clearly more research is needed in this area. Future studies should address the dosing, pharmacokinetics, and mechanism of action of ribavirin. The rationale for choosing a target concentration of 15 mol/L appeared to be arbitrary and not based on good pharmacokinetic and pharmacodynamic data. Lower plasma concentrations may have been as effective but it also raises the question as to whether ribavirin should continue to be administered based on body weight or dosed to achieve a target serum concentration. Another possibility is that inter-individual variations in the metabolism of ribavirin may account for differences in clinical efficacy. Thus, patients who are nonresponders to weight-based therapy may be receiving subtherapeutic doses of ribavirin. This possibility may to a certain extent explain the low SVR rates observed in two trials of peginterferon and ribavirin in human immunodeficiency virus coinfected patients[12][13] and a recent Spanish Trial of 72 weeks versus 48 weeks of peginterferon plus ribavirin for patients who fail to achieve virological clearance at week four.[14]
In summary, Lindahl et al.'s article is an important study whose results probably provoke more thought about ribavirin's mechanism of action than provide a practical therapeutic approach for patients with chronic hepatitis C. At present, this strategy should be reserved for the setting of controlled clinical trials and not become part of standard clinical practice.
References
1 Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001; 358: 958-965. Links
2 Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002; 347: 975-982. Links
3 Food and Drug Administration Center for Drug Evaluation and Research Meeting of the Antiviral Drugs Advisory Committee. www.fda.gov/ohrms/dockets/ac/01/briefing/3819b1_03_FDA-Clinical%20review.htm 2003.
4 Hadziyannis SJ, Sette H Jr, Morgan TR, Balan V, Diago M, Marcellin P, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med 2004; 140: 346-355. Links
5 Dalgard O, Bjoro K, Hellum KB, Myrvang B, Ritland S, Skaug K, et al. Treatment with pegylated interferon and ribavarin in HCV infection with genotype 2 or 3 for 14 weeks: a pilot study. HEPATOLOGY 2004; 40: 1260-1265. Links
6 Lindahl K SL, Bruchfeld A, Schvarcz R. High dose ribavirin in combination with standard dose peginterferon for treatment of chronic hepatitis C patients. HEPATOLOGY 2005; 41: 275-279. Links
7 Muir AJ, Bornstein JD, Killenberg PG. Peginterferon alfa-2b and ribavirin for the treatment of chronic hepatitis C in blacks and non-Hispanic whites. N Engl J Med 2004; 350: 2265-2271. Links
8 McHutchison JG, Poynard T, Pianko S, Gordon SC, Reid AE, Dienstag J, et al. The impact of interferon plus ribavirin on response to therapy in black patients with chronic hepatitis C. The International Hepatitis Interventional Therapy Group. Gastroenterology 2000; 119: 1317-1323. Links
9 Sullivan SD, Jensen DM, Bernstein DE, Hassanein TI, Foster GR, Lee SS, et al. Cost-effectiveness of combination peginterferon alpha-2a and ribavirin compared with interferon alpha-2b and ribavirin in patients with chronic hepatitis C. Am J Gastroenterol 2004; 99: 1490-1496. Links
10 Gish RG, Arora S,, Nelson D, Fried MW, Reddy KR, Xu Y, et al. End-of-treatment (EOT) response in therapy-naïve patients treated for hepatitis C with viriamidine in combination with pegylated interferon alfa-2a [Abstract]. HEPATOLOGY 2004; 40: 388A. Links
11 Pockros PJ, Pessoa MG, Diago M, de Lôurdes Candolo Martinelli A, Berg T, Germanidis, et al. Combination of levovorin (LVV) and peginterferon alfa-2a (40Kd) Pegasys®) fails to generate a virological response comparable to ribavrin (RBV, Copegus®) and peginterferon alfa-2a (40Kd) in patients with chronic hepatitis C [Abstract]. HEPATOLOGY 2004; 40: 391A. Links
12 Torriani FJ, Rodriguez-Torres M, Rockstroh JK, Lissen E, Gonzalez-Garcia J, Lazzarin A, et al. Peginterferon Alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med 2004; 351: 438-450. Links
13 Chung RT, Andersen J, Volberding P, Robbins GK, Liu T, Sherman KE, et al. Peginterferon alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med 2004; 351: 451-459. Links
14 Sanchez-Tapias JM, Diago M, Escartin P, Enriquez J, Moreno R, Romero-Gomez M, et al. Longer treatment duration with peginterferon alfa-2a (40KD) (Pegasys®) and ribavirin (Copegus®) in naïve patients with chronic hepatitis C and detectable HCV RNA by week 4 of therapy: Final results of the randomized, multicenter TERAVIC-4 study [Abstract]. HEPATOLOGY 2004; 40: 218A. Links
Article Text
Although improvements have been made in the antiviral therapy of chronic hepatitis C virus (HCV), patients with genotype 1 infection with a high level of HCV RNA still pose a therapeutic challenge. The standard combination treatment with 12 months of peginterferon combined with ribavirin results in a sustained virological response rate of approximately 40% for this subset of patients.[1-3]
The mode of action of ribavirin is not firmly established.[4] Furthermore, data regarding the correlation between the dose and the resulting concentration of ribavirin are limited, and the optimal dose is not known. For the treatment of chronic HCV, ribavirin is currently dosed according to body weight, usually between 800 and 1,200 mg daily.[5] In a recent report by Hadziyannis et al.,[3] patients with genotype 1 and a high viral load responded better to a ribavirin dose of 1,000 to 1,200 mg daily than to 800 mg/day combined with peginterferon.[3] In contrast to current recommendations, we have recently shown that renal function is of greater importance than body weight for ribavirin clearance, and we therefore have suggested the use of a pharmacokinetic formula based primarily on renal function as a tool to determine the accurate dose of ribavirin for treatment.[6]
In a previous study, we have shown that with a standard ribavirin treatment dose of 800 to 1200 mg daily, the mean ribavirin concentration was 8.2 umol/L (range, 0-17.7).[7] Other studies have shown a correlation between the ribavirin concentration and viral response.[8][9] We propose that concentrations greater than 15mol/uL may improve the response rate for genotype 1 patients infected with HCV.
The aim of this study was to evaluate safety of and tolerance to a high dose of ribavirin that is selected and adjusted to achieve a high steadystate concentration of ribavirin (>15 umol/L). We also aimed to prospectively evaluate the use of a pharmacokinetic formula to predict the initial dose of ribavirin required to reach the intended plasma concentration of ribavirin and to measure the viral response to this dose. For this purpose, we conducted an open label study on patients with chronic HCV, genotype 1 and high viral load, with one treatment arm of ribavirin dosed individually, in combination with a standard dose of peginterferon alfa-2a, for 48 weeks.
Patients and Methods
Patients.
Ten previously untreated patients with chronic HCV, genotype 1 and a viral load of >800 000 International Units (IU)/mL, who were attending the outpatient clinic at the Department of Infectious Diseases, Karolinska University Hospital Huddinge, were enrolled in this open-label, prospective trial. The inclusion criteria were as follows; age >18 years, elevated alanine aminotransferase, positive anti-HCV antibody test, detectable serum HCV RNA, and a liver biopsy consistent with chronic HCV but without cirrhosis. Patients with other forms of liver disease, active hepatitis A or hepatitis B infection, hepatocellular carcinoma, human immunodeficiency virus infection, anemia, a previous diagnosis of severe depression or other psychiatric disease, significant cardiac disease, renal disease, seizure disorders, severe retinopathy, or pregnancy were excluded from the study.
Liver biopsies were classified according to inflammation (grade) and fibrosis (stage) on a noncontinuous scale of 0 to 4.[10]
The study was approved by the local ethical review board, and all patients gave their informed consent.
Treatment.
The treatment consisted of 180 ug peginterferon alfa-2a (Pegasys, Roche AB, Stockholm, Sweden) subcutaneously once weekly, in combination with ribavirin for 48 weeks. Ribavirin was administered orally with food as 200-mg capsules/tablets, twice or three times daily. Initially the patients were given Rebetol (Schering-Plough AB, Stockholm, Sweden); that is, the first five patients included in the study were treated with Rebetol. Copegus (Roche AB) then became available and was judged to be favorable with regard to compliance because of the smaller size of the tablet, considering the large number of tablets that needed to be taken by the patient. An estimated 90% or more of the ribavirin treatment given throughout the study was Copegus. The change of study drug was decided to be acceptable because the treatment was concentration controlled and not dose controlled; hence, bioequivalence was not an issue.
The initial ribavirin dose was individualized and calculated from a pharmacokinetic formula that is primarily based on renal function and aims to achieve a steady-state concentration of ribavirin of >15 mol/L.[6] When necessary, the dose of ribavirin was increased to reach the target concentration, as indicated by the measured plasma ribavirin concentrations. Creatinine clearance was estimated according to the Cockroft-Gault equation.[6] Treatment was discontinued in patients who had detectable virus (>600 IU/mL) after 24 weeks of treatment.
Monitoring.
The patients were monitored every 2 to 4 weeks during the entire 48-week treatment period and were followed for an additional 24 weeks, after cessation of treatment. General monitoring included full blood cell counts and hemoglobin level, serum alanine aminotransferase, serum urate, thyroid function tests, and weight.
Plasma samples were collected approximately 12 hours after the dose administration, and ribavirin concentrations were measured by high-performance liquid chromatography[11] every 2 to 4 weeks during the first 24 weeks of treatment. During the final 24 weeks of treatment, the ribavirin concentration was measured every 8 weeks or more frequently if dose adjustments were made.
HCV genotyping was done before inclusion by using an in-house method.[12] Qualitative (sensitivity <50 IU/mL, Cobas Ampliprep/Cobas Amplicor, HCV test version 2.0, Roche Molecular Systems Inc., Branchburg, NJ) or quantitative (sensitivity <600 IU/mL, Cobas Ampliprep/Cobas Amplicor HCV Monitor version 2.0, Roche Molecular Systems Inc.) HCV polymerase chain reaction tests were conducted at study weeks 0, 4, 12, 24, 48, and 72.
Safety Aspects.
In patients with moderate to severe anemia, treatment options included blood transfusion and reduced treatment doses. Ribavirin was discontinued if the hemoglobin level fell below 8.0 g/dL. To prevent severe anemia, erythropoietin (Neorecormon, Roche AB) and oral iron supplement were used. Dose adjustments of peginterferon were made according to well-established guidelines.[1][2]
Results
Ribavirin Dosing.
Ten patients, seven men and three women, mean age 51 years (range, 40-64) were enrolled between November 2002 and April 2003. The patient characteristics are shown in Table 1.
The initial daily dose of ribavirin was calculated from the pharmacokinetic formula, which aimed to achieve a steady-state concentration of ribavirin of >15 umol/L (see Patients and Methods section) and resulted in a mean dose of 1,520 mg/day (range, 1,200-2,200), as shown in Table 2. However, the mean concentration at treatment week 4 was 8.6 umol/L (range, 6.0-15.7). After a gradual dose escalation, the mean daily dose of ribavirin at treatment week 24 was 2,540 mg/day (range, 1,600-3,600), which in turn resulted in a mean ribavirin concentration of 14.7 (range, 7.8-22.0). The highest individual dose given was 4,000 mg daily.
|
|
| |
| |
 |
|
| |
| |
|
|
| |
| |
 |
|
| |
| |
AUTHOR DISCUSSION
Patients with chronic HCV, genotype 1 and high viral load, who were the target group of this study, have a moderate expected outcome with standard combination treatment; improvements in treatment efficacy are needed.[1-3] Our hypothesis was that the fraction of patients with sustained virological response could increase with high-dose ribavirin treatment, resulting in high plasma concentration levels. To investigate this, we conducted this pilot study with the primary goal of evaluating safety and tolerance to treatment.
The optimal target concentration for ribavirin is not well established. In a previous study, we found that patients given standard ribavirin dosing, 800 to 1,200 mg daily, had a mean ribavirin plasma concentration of 8.2 umol/L.[7] The probability of response to treatment has been shown to increase with increasing ribavirin concentrations.[8][9] Therefore, we chose a target concentration of >15 umol/L, based partly on the data of Jen et al.[8] and partly on our prior studies in which some patients on standard therapy achieved concentrations above 15 mol/L. We reasoned that the more effective concentrations achieved in a small proportion of patients on standard therapy should be explored under controlled conditions.
Increases in ribavirin doses from current standard regimens (800-1,200 mg daily) have been discussed. However, the use of high doses of ribavirin as in the current study (1,600-4,000 mg daily) has not to our knowledge been described for treatment of chronic HCV.
Some major limitations of this study were the small number of patients, the lack of controls, and that patients with cirrhosis were excluded. The side effects were more frequent and serious, in particular potentially life-threatening anemia, than those observed with standard combination treatment. However, only minor treatment interruptions occurred among the ten patients who were treated with doses of ribavirin substantially exceeding currently used standard doses.
As expected, anemia was the most challenging side effect to manage. We have previously shown that the degree of anemia is correlated to the plasma ribavirin levels.[7] This result was verified in our study, since the patients who were exposed to very high ribavirin concentrations developed severe anemia. In two patients, the hemoglobin dropped below 8.0 g/dL, and they were treated with blood transfusions and discontinuation of ribavirin for a short period. The hemoglobin level dropped to below 10.0 g/L in five patients. Other measures used to limit anemia were minor dose reductions in ribavirin dose and treatment with erythropoietin. Other side effects also seemed to be more frequent and more severe than with standard treatment, in particular fatigue, nausea, and pruritus. Our impression was that this treatment regimen was more demanding for the patients than the standard treatment. Because of the extent and severity of the side effects, closer monitoring and extensive interventions were needed. However, by using these measures to correct anemia and treat other side effects, it was possible, with few exceptions, to maintain the patients on treatment with high doses of ribavirin.
The primary goal of this small pilot study was to determine feasibility and safety of the treatment, and not virological outcome. However, in this difficult-to-treat patient population with genotype 1 and a high viral load, nine of ten patients were cured by standard definitions, which seems to be a better response than that found in studies using standard ribavirin doses.[1-3] Absence of patients with cirrhosis in our study population is a potential selection bias. According to current opinion, the major action of ribavirin is to prevent relapse. Interestingly, our impression was that the virological response increased with time and correlated with increasing ribavirin concentrations. Further studies are needed to investigate whether high ribavirin doses could enhance the primary viral response.
An important part of this work was to prospectively analyze the validity of a pharmacokinetic formula, based primarily on renal function, that calculates the dose of ribavirin for any given target concentration.[6] Unexpectedly, the formula systematically underestimated the dose of ribavirin. Thus, in general, it was necessary to gradually increase the dose of ribavirin during the first part of the treatment to achieve the chosen target concentration. As a consequence, it took considerably longer to achieve steady state in this study than with the common regimen using the same dose of ribavirin over the entire treatment period. The reasons the formula overestimated the expected concentration of ribavirin for a given dose are being investigated. One possibility is that a small number of samples inappropriately stored in the previous study[6] may have biased the model. It is also possible that the absorption of ribavirin is saturable. A third possibility, assuming a difference in the bioavailability of different ribavirin products, is that the use of Copegus in this study instead of Rebetol which was used in the population pharmacokinetic analyses,[6] could contribute to the nonoptimal performance of the formula. However, we find this explanation unlikely - firstly, because a recent small study has shown no difference in bioavailability between the two products (bioequivalence study of ribavirin formulations Rebetol and Ro 20-9963 administered orally to individuals with current or previous chronic hepatitis C infection, 2001, Roche, data on file). Secondly, the first two patients in our study received Rebetol during the first 16 weeks with a similar underestimation of ribavirin dose (calculated by the formula) as in the patients treated with Copegus.
In conclusion, we have shown in this small pilot study that it is feasible to treat patients with chronic HCV with high doses of ribavirin without any major treatment interruptions. Side effects were more frequent and serious, in particular potentially life-threatening anemia, than those usually observed with standard combination treatment. Therefore, we do not recommend this treatment regimen outside clinical trials. In particular, patients with coronary heart disease and patients with cirrhosis would be at great risk. The pharmacokinetic formula used to calculate the initial ribavirin dose systematically underestimated the dose. It is still a useful tool, but it needs to be revised. Considering the high proportion of patients cured in this pilot study, additional studies would be of great value to further evaluate safety and side effects as well as the viral efficacy in a controlled fashion. Nonresponders to conventional therapy may be the appropriate target population for future studies.
References
1 Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001; 358: 958-965. Links
2 Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002; 347: 975-982. Links
3 Hadziyannis SJ, Sette H Jr, Morgan T, Balan V, Diago M, Marcellin P, et al. Peginterferon alfa-2a and ribavirin combination therapy in chronic hepatitis C. Ann Intern Med 2004; 140: 346-355. Links
4 Lau JY, Tam RC, Liang TJ, Hang Z. Mechanism of action of ribavirin in the combination treatment of chronic HCV infection. HEPATOLOGY 2002; 35: 1002-1009. Links
5 Jen J, Laughlin M, Chung C, Heft S, Affrim MB, Gupta SK, et al. Ribavirin dosing in chronic hepatitis C: application of population pharmacokinetic-pharmacodynamic models. Clin Pharmacol Ther 2002; 72: 349-361. Links
6 Bruchfeld A, Lindahl K, Schvarcz R, Stahle L. Dosage of ribavirin in patients with chronic hepatitis C should be based on renal function: a population pharmacokinetic analysis. Ther Drug Monit 2002; 24: 701-708. Links
7 Lindahl K, Schvarcz R, Bruchfeld A, Stahle L. Evidence that plasma concentration rather than dose per kg bodyweight predict ribavirin induced anaemia. J Viral Hepatol 2004; 11: 84-87. Links
8 Jen JF, Glue P, Gupta S, Zambas D, Hajian G. Population pharmacokinetic and pharmacodynamic analysis of ribavirin in patients with chronic hepatitis C. Ther Drug Monit 2000; 22: 555-565. Links
9 Larrat S, Stanke-Labescque F, Plages A, Zarski JP, Bessard G, Souvignet C. Ribavirin quantification in combination treatment of chronic hepatitis C. Antimicrob Agents Chemother 2003; 47: 124-129. Links
10 Schvarcz R, Glaumann H, Reichard O, Weiland O. Histological and virological long-term outcome in patients treated with interferon-alpha2b and ribavirin for chronic hepatitis C. J Viral Hepatol 1999; 6: 237-242. Links
11 Svensson J-O, Bruchfeld A, Schvarcz R, Ståhle L. Determination of ribavirin in serum using highly selective solid-phase extraction and high performance liquid chromatography. Ther Drug Monit 2000; 22: 215-218. Links
12 Yun Z, Lara C, Johansson B, Lorenzana RI, Sonnerborg A. Discrepancy of hepatitis C virus genotypes as determined by phylogenetic analysis of partial NS5 and core sequences. J Med Virol 1996; 49: 155-160. Links
|
|
| |
| |
|
|
|