| |
HIV and Hepatitis C Virus Coinfection Update
|
| |
| |
Arthur Y. Kim, MD and Raymond T. Chung, MD
Department of Medicine, Massachusetts General Hospital, 55 Fruit Street, Boston, MA 02114, USA.
Current Hepatitis Reports August 2004
Sharing routes of transmission, hepatitis C virus (HCV) and human immunodeficiency virus type 1 (HIV-1) are often harbored in the same host, establishing chronic infections characterized by high serum viral loads. HIV-1 impacts the course of HCV infection by increasing the rate of HCV viral persistence, quantitative viral loads, and liver fibrosis progression rate. HCV in turn affects HIV management, particularly by increasing the risk of hepatotoxicity. Future studies will focus on understanding the pathogenesis of accelerated liver fibrosis in HIV-infected individuals, the natural history of HCV in the era of antiretroviral therapy, and the principles of managing these two infections within the same individual.
Introduction
Hepatitis C virus (HCV)/human immunodeficiency virus type 1 (HIV-1) coinfection is a frequently encountered scenario due to common modes of transmission. Although the disease burden of other opportunistic infections has declined in the era of effective antiretroviral therapy, HCV has emerged as a frequent cause of morbidity and mortality for HCV/HIV-1-coinfected subjects. The goal of this article is to update practitioners on special issues facing this population, highlighting both the accelerated natural history of HCV in HIV-infected individuals and recent data that influence the complex management of coinfection with these two viruses.
Epidemiology
Both HIV-1 and HCV are acquired parenterally, sexually, or vertically, but HCV is most efficiently transmitted by bloodborne routes, such as intravenous drug use or by transfusion of infected blood products. Depending on the distribution of route of acquisition, HCV prevalence rates can vary widely in cohorts of HIV-1-infected individuals. An overall 16% rate of HCV antibody prevalence was detected within a cross-sectional analyses of HIV-positive subjects entering clinical trials [1, 2], whereas approximately 45% of subjects analyzed within a US inner-city academic center were HCV seropositive [3]. Conversely, approximately 10% of HCV-infected individuals also are infected with HIV-1, although this rate can be as high as 25% depending on the particular cohort [4]. Overall, although the prevalence of HCV in the United States is estimated at 1.8%, or 3.9 million persons [5], approximately 300,000 individuals are estimated to be coinfected with both viruses.
Natural History
Several lines of evidence implicate HIV-1 as a cofactor that accelerates the natural history of HCV. Given the key role of the immune system in containing HCV, it is not surprising that levels of HCV viremia are higher in HIV-1-coinfected patients than in those infected with HCV alone [6]. These findings mirror those in other immunocompromised populations, such as subjects undergoing liver transplantation. However, no clear correlation of HCV-RNA levels with CD4+ cell counts exists, nor has a definite relationship between levels of HCV and HIV-1 viremia been established.
HIV-1 coinfection is associated with more rapid progression of HCV fibrosis, compared with HCV alone, particularly as CD4 T-cell counts decline [7, 8, 9]. It has been observed in multiple cohorts that progression to liver failure is accelerated during HCV/HIV-1 [10], with a negative impact on hospital admission and death rates [11, 12]. The relative contribution of HCV to morbidity and mortality has risen in the era of highly active antiretroviral therapy (HAART), reflecting a postponement of HIV-related opportunistic diseases and enhanced progression of HCV-related liver disease. (note from Jules Levin: recently published studies, since this article, report findings that suggest that judiciously timed HAART & undetectable HIV RNA & increased CD4 counts might slow HCV disease progression in coinfected patients due to improved immune system. I think this question remains unresolved & needs further examination; many variables may contribute to HCV disease progression).
Reciprocal effects of HCV on HIV-1 disease progression have been difficult to detect [13, 14]. Some studies indicate that coexisting HCV may blunt the immunologic response or rise in CD4 T-cell count while on HAART and may be associated with HIV-1 disease progression [15*, 16], although others have not verified this phenomenon [17*, 18]. Activation of CD4 T cells and their sensitization to apoptosis induced by HCV are potential mechanisms for the dampened immunologic response [19, 20].
The natural history of HCV in coinfected subjects during the era of HAART is being elucidated. Although definitions of hepatotoxicity vary, studies have consistently shown that liver enzyme elevations while on HAART are more frequent in coinfected persons, with an approximately threefold increased risk of hepatotoxicity (Table 1).
|
|
| |
| |
 |
|
| |
| |
Nevirapine, which has been related with serious liver toxicity in otherwise healthy individuals [29], is associated with both a higher rate of liver enzyme elevation and increased fibrosis progression in coinfected persons [27, 30, 31]. Additional hepatic-related toxicities associated with both antiretroviral therapy and HCV independently include metabolic syndromes such as insulin resistance and hepatic steatosis [32, 33], often associated with lipodystrophy.
Although the shorter-term risks of hepatotoxicity are well documented, the longer-term outcomes of HAART in this population remain under investigation. Studies have not observed increased inflammation or fibrosis progression rates in coinfected subjects on HAART [9, 34], whereas one study found a protective benefit of protease inhibitor-based therapy on liver histology [35]. (note from Jules Levin: since this report was published Mark Sulkowski et al published a study reporting: "....We found no evidence for the hypothesis that ART causes chronic histological liver disease. Rather, we found the long-term liver enzyme pattern to be a much stronger predictor of eventual liver fibrosis....". Link to full report:
"The effect of antiretroviral therapy on liver disease among adults with HIV and hepatitis C coinfection"
http://www.natap.org/2005/HCV/010305_05.htm
Hepatotoxicity may be due to enhanced drug toxicity, increased HCV replication, or enhancement of the immune response. There are several lines of clinical and experimental evidence examining the question of immune reconstitution against HCV in HIV-positive persons, but understanding of the potential mechanisms remains elusive. It is clear that the vast majority of subjects who have been studied longitudinally on HAART do not experience a reduction in viral load [36*], and case reports of hepatic decompensation following the institution of HAART are also of concern [37, 38]. Emerging reports of sustained clearance of HCV after immune reconstitution on successful antiretroviral therapy (without exogenous interferon [IFN]) strongly suggest that successful immunity can be restored [39, 40, 41, 42, 43]. Therefore, immune reconstitution to HCV is potentially harmful for some and, rarely, beneficial. Nevertheless, the potential benefits of HAART in preventing mortality due to the reduction of opportunistic infections in balance appears to outweigh the risks of severe hepatotoxicity in coinfected persons [44*].
Pathogenesis
Unfortunately, little is understood about the mechanisms underlying the higher viral loads, hepatotoxicity, and accelerated liver disease observed in HIV-1/HCV-coinfected individuals. Because HCV viral loads are higher in HIV-positive individuals, it remains possible that increased cytopathogenicity of the virus contributes to some cases of accelerated liver disease. The finding of the fibrosing cholestatic variant of HCV in HIV-1 coinfection [45] supports this concept, but is likely to only account for extreme cases. Direct virologic interaction is unlikely, because HIV-1 does not directly infect hepatocytes but targets macrophages and CD4+ T lymphocytes.
Another proposal is that CD4+ T-cell depletion due to HIV-1 results in abrogation or dysregulation of the host immune response, which has been implicated in both initial clearance of the virus and in disease pathogenesis [46]. The remarkable ability of HCV to establish chronic infection is likely related to the inability of the host immune response to achieve viral clearance after acute infection [47]. Innate immunity, such as type 1 IFNs, may be circumvented by inhibiting genes that stimulate IFN production, by inhibiting IFN signaling, or by blocking IFN-inducible protein kinases. Although the adaptive immune response also contributes to clearance of the virus, several pathways of abrogating or eluding this arm of the immune system have been described. Alterations in lymphocyte cytokine expression in the local hepatic microenvironment may contribute to tissue injury in HIV-1-positive individuals. The correlates of immune reconstitution are being actively investigated, including analysis of both the innate and adaptive immune responses.
(note from Jules Levin: since this article was published Chung published study reporting:"... The finding that HCV-specific CD8+ T-cell responses decline with diminishing absolute CD4+ counts provides a possible explanation for the more rapid HCV disease progression in the setting of HIV-1 coinfection..." In other words, when low CD4 count is present there is less stimulation of CD8 HCV cells which target HCV. This may contribute to quicker HCV progression in HIV+ individuals..."
Link to full report:
CD4 Deficiency May Explain HIV Affect on HCV
http://www.natap.org/2005/HCV/012005_02.htm
Diagnosis and Evaluation
All individuals being evaluated for HIV-1 should also be screened for HCV infection [48]. The goals are to identify those at risk for HCV-related complications and possible candidates for treatment. The most often used and reliable test is the third-generation serologic enzyme immunoassay test for antibodies against HCV. Confirmatory tests include both a recombinant immunoblot assay (HCV-RIBA) and HCV-RNA testing.
Practitioners should be aware of three situations in which the initial enzyme-linked immunosorbent assay may be negative despite prior infection with HCV: 1) loss of antibody response years after seroconversion following clearance of the virus [49]; 2) loss of antibody responses when severely immunosuppressed (CD4 counts < 100 cells/mm3); and 3) during acute infection prior to seroconversion. For the latter two reasons, it is advisable to consider HCV-RNA testing after a negative antibody in certain subsets of HIV-1-positive individuals, especially when liver function tests are elevated in the absence of other causes or if epidemiologic and clinical history suggest HCV infection, especially in the acute phase.
Qualitative HCV-RNA levels are recommended to confirm current HCV infection, although because RNA levels can occasionally be detected intermittently, diagnostic and treatment decisions should not be based on the results of a single HCV-RNA assay. Unlike HIV-1 RNA plasma levels, the quantity of HCV RNA does not predict either the extent or rate of progression of disease but helps to determine the probability of treatment response. Therefore, quantitative HCV-RNA testing should be reserved for situations in which antiviral treatment is being considered.
Further evaluation of coinfected persons who are viremic with HCV involves determination of the fibrosis stage, which helps to inform both the timing and efficacy of treatment. Physical examination and laboratory testing (eg, prothrombin time, albumin, and platelet count) may reveal the presence of cirrhosis, but cannot assess earlier stages of fibrosis. Although noninvasive means of predicting liver fibrosis are under investigation, the current gold standard for assessing the extent of hepatic disease is biopsy, which provides prognostic information and identifies candidates for treatment. Importantly, histologic data can identify the subject in whom minimal disease is present and in whom treatment can be deferred. For viremic individuals who defer treatment, repeat biopsy in 3 to 5 years can be considered. Because antiretroviral therapy has elicited hepatic decompensation in subjects with either advanced fibrosis or cirrhosis, liver biopsy should also be considered in coinfected persons who are candidates for antiretroviral therapy.
Management
The management of HCV-related liver disease is complex in coinfected persons and requires careful knowledge of the drugs used to treat both infections. For coinfected persons viremic with HCV, consideration of IFN-based treatment should include individualized factors such as likelihood of progression of liver disease, probability of successful treatment, tolerability of therapy and impact on quality of life, and risk of additive toxicities with antiretroviral or other medications.
The first steps in managing this population include reduction of alcohol consumption and illicit drug abuse, both of which have independent negative effects on the liver and to be in compliance with therapy. In seronegative persons, vaccinations for hepatitis A and B are recommended to prevent the potentially devastating effects of a superimposed hepatitis.
Treatment
Antiretroviral treatment
In situations of severe CD4 depletion due to HIV-1, it is generally advisable to institute HIV-1 therapy to achieve immunologic benefit against other opportunistic infections [50, 51]. Overall, most coinfected individuals do not experience hepatotoxicity on HAART; therefore, the increased risk of this toxicity in HCV-positive patients should not by itself represent a reason to withhold anti-HIV-1 therapy. However, in selected situations anti-HCV treatment may be necessary to allow tolerance of antiretrovirals [52, 53]. In particular, for subjects with HIV-1 viremia who do not yet meet criteria for initiation of antiretroviral therapy, it remains unanswered whether one should consider HIV-1 treatment or HCV treatment first. Studies are underway that will address the appropriate temporal sequence of antiviral therapy for this population.
When choosing a regimen, nevirapine may be avoided for the HCV-positive individual due to increased hepatic risks, based on current evidence. Risk may also be increased if exposed to higher-dose ritonavir, now rarely used, but should be minimized with the lower doses typically used to boost other protease inhibitors [54]. If IFN therapy is a future possibility, an alternative to didanosine (ddI) may be chosen due to potential interactions with ribavirin (RBV) (see below). In any event, strict follow-up to monitor for antiretroviral-related toxicities, including both liver enzyme elevation and lactic acidosis, is warranted for this population. Understanding of the effects of HCV on the in vivo metabolism of these drugs should be elucidated by studies of therapeutic drug monitoring.
IFN-based therapies
Advances in the treatment of HCV in monoinfected persons are rapidly being translated to those coinfected with HIV-1. The goals of treatment are twofold: to eradicate virus and to halt histologic progression. Prior trials of traditional IFN with or without RBV achieved disappointing success rates (sustained virologic response [SVR]) of approximately 18% to 40% in coinfected persons [55, 56, 57, 58, 59]. Recently, the results of three large randomized controlled trials of peginterferon (PEG) versus standard IFN in coinfected subjects have been reported and are summarized in Figure 1 and Tables 2 and 3.
|
|
| |
| |
 |
|
| |
| |
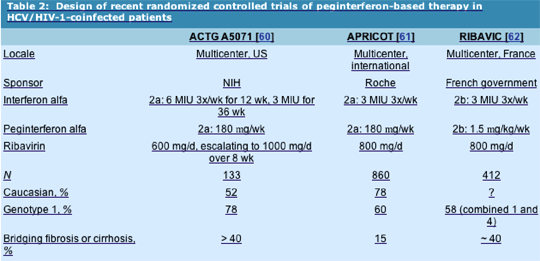
ACTG A5071
Design: The ACTG A5071 [60] was a US-based, National Institutes of Health-sponsored study that randomized 133 HCV/HIV-1-coinfected patients to one of two arms: peginterferon alfa-2a 180 mg weekly or IFN alfa-2a 6 MIU three times a week for 12 weeks followed by 3 MIU three times a week for 36 weeks. Because of prior experiences with high dropout rates for anemia in the HIV-1-coinfected population, each arm received RBV in a dose-escalation schedule of 600 mg/d for 4 weeks followed by 800 mg/d for 4 weeks and then followed by 1000 mg/d as a ceiling dose. All subjects underwent baseline liver biopsy. Because of safety concerns with this combination, subjects were evaluated at week 24 for virologic response. Those subjects who failed to experience virologic clearance were required to undergo liver biopsy. Subjects experiencing histologic response (³ two-point reduction in histologic activity index, Ishak scale) continued the study drug; those refusing biopsy or those who failed to experience histologic response stopped at week 24. Virologic responders continued the drug until week 48, at which time they underwent liver biopsy.
Inclusion criteria: HCV-RNA positive, any alanine aminotransferase (ALT) (normal or elevated), no decompensated cirrhosis, HIV-1 RNA level less than 10,000 copies/mL with CD4+ cell count greater than 100 cells/mm3 on stable antiretroviral therapy, or CD4+ cell count greater than 300 cells/mm3 with any HIV-1 RNA level.
Population: Half the cohort was Caucasian, the remainder African-American (33%) or Hispanic (15%). Median CD4+ cell counts were 444 and 492 cells/mm3 in the IFN/RBV and PEG/RBV arms, respectively, and 86% were on antiretroviral therapy. Baseline HIV-1 RNA levels were less than 50 copies/mL, 78% had genotype 1 HCV, and over 40% had bridging fibrosis or cirrhosis.
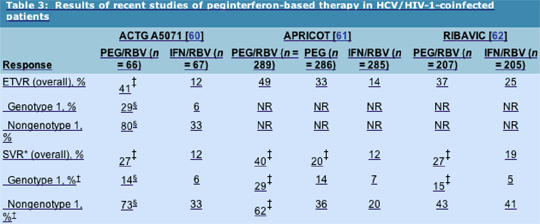
Results: Overall, a significantly higher proportion of the PEG/RBV arm achieved end-of-treatment virologic response (ETVR) (41% vs 12%; P < 0.001) and SVR (27% vs 12%; P = 0.03). The proportions achieving ETVR and SVR were also significantly higher among nongenotype 1 patients compared with genotype 1 patients (P < 0.001).
Histologic improvement was seen in 54% of virologic responders and 36% of week 24 virologic nonresponders. Although there was a higher frequency of grade 4 events in the PEG/RBV arm, only 12% in each arm prematurely discontinued the study drug, suggesting that each regimen and the RBV dose escalation schedule was well tolerated. Absolute CD4+ cell counts fell with treatment but reverted to baseline off therapy. No AIDS progression or loss of HIV-1 control was observed. The negative predictive value of week 12 early virologic response (failure to clear or decrease HCV-RNA level by greater than 2 log10 from baseline) was 100% for failure to achieve SVR.
APRICOT
Design: The APRICOT (AIDS Pegasys Ribavirin International Co-infection Trial) [61] was a large, multicenter, international, Roche-sponsored trial that randomized nearly 900 patients into one of three arms for 48 weeks: 1) IFN alfa-2a 3 MIU three times a week with RBV 800 mg/d; 2) peginterferon alfa-2a 180 mg weekly plus placebo; or 3) peginterferon alfa-2a 180 mg weekly plus RBV 800 mg/d.
Population: A total of 860 subjects received at least one dose of the study drug. Patients were required to have a CD4+ cell count greater than 100 cells/mm3 and an elevated ALT level and were also required to be HCV-RNA positive. Seventy-eight percent were Caucasian, 15% had cirrhosis, and 60% were classified as genotype 1. The mean CD4+ cell count was greater than 500 cells/mm3 in each arm, and two thirds had undetectable HIV-1 RNA levels.
Results: The proportion of peginterferon/RBV recipients achieving SVR was significantly higher than that in the IFN/RBV or peginterferon alone arms (40% vs 12% vs 20%, respectively; P < 0.0001). The rate of SVR in the peginterferon alone arm was significantly higher than that in the IFN/RBV arm (P = 0.0078).
Premature dropout rates for adverse events were 15% to 16% across all three arms. Absolute CD4+ cell count fell, but the relative CD4+ percentage did not. HIV-1 RNA level was reported to fall by a 0.9 log in subjects receiving peginterferon alfa-2a, but no change was seen in those receiving IFN alfa-2a.
RIBAVIC
Design: The RIBAVIC [62] was a French government-sponsored trial that randomized 205 HCV/HIV-1-coinfected subjects to receive peginterferon alfa-2b 1.5 mg/kg weekly plus RBV 800 mg/d and 207 subjects to receive IFN alfa-2b 3 MIU three times a week plus RBV 800 mg/d for 48 weeks.
Population: Patients were included who had CD4+ cell counts greater than 200 with stable HIV-1 RNA levels, and detectable HCV RNA. Two thirds had undetectable HIV-1 RNA, and 82% were on HAART. Mean CD4+ cell count was 514 cells/mm3. HCV genotypes were 1 or 4 in 58%, 3 in 34%, with 22% to 25% having bridging fibrosis and 14% to 18% cirrhosis.
Results: The rate of SVR was significantly higher among peginterferon/RBV recipients compared with those treated with IFN/RBV (27% vs 19%; P = 0.03). Dropout rates were high, with 31% dropping out due to adverse events, mostly psychiatric events, and increases in lactate levels associated with ddI. Virologic responders experienced an improvement in activity (inflammation) score on follow-up liver biopsy, whereas nonresponders experienced little improvement.
These three trials clearly demonstrate the superiority of peginterferon alfa and RBV over standard IFN-based therapies. Although overall SVRs (27% to 40%) were disappointingly lower than historic large trials of peginterferon alfa in monoinfected individuals, they demonstrate that a significant number of HIV-1-positive persons can successfully undergo HCV treatment. Unfortunately, the SVR rates for genotype 1-infected person sharply contrasted with those seen for nongenotype 1-infected persons. This difference appears to be largely driven by a high relapse rate in genotype 1-infected subjects. Overall safety was acceptable, with transient CD4+ T-cell count reductions observed in all three cohorts without an increase in HIV-1-related events.
Differences in trial design and patient population may explain the disparity in SVRs between the APRICOT study and ACTG A5071 and RIBAVIC. First, the dosing regimen of RBV in the ACTG trial involved initial dosing of 600 mg/d, increasing to 1000 mg/d over the first 8 weeks if hematologic parameters allowed. Although this schedule was designed to lower dropout rates, it may have adversely affected the relapse rate and SVR rate. Second, differences in patient population were noted (Table 2): both A5071 and RIBAVIC enrolled a relatively larger proportion of subjects with advanced fibrosis, and A5071 enrolled a significant proportion of African-American subjects, both factors associated with lower SVR rates. Further study of RBV dose optimization in this population, especially those with genotype 1 infection, is warranted. The use of epoetin alfa may particularly play a role in maintaining dose in HCV-positive/HIV-1-negative persons [63]. Although a 48-week regimen of peginterferon and RBV can be recommended for genotype 2 and 3 infection based on the trial data, the efficacy of a shorter treatment course in coinfected subjects is not yet determined in this group. The use of early virologic response determinations may be particularly useful in genotype 1-coinfected persons, for whom SVR rates are low.
Finally, although SVR is the primary goal, a secondary benefit of IFN-based therapies may be histologic benefit in the absence of SVR. A5071 reported histologic response in a significant portion of virologic nonresponders, providing a rationale for the study of the potential clinical benefits of longer-term maintenance IFN regimens in HIV-1-positive individuals.
In summary, peginterferon alfa and RBV in combination now represent the current standard in treatment of HIV-1-positive individuals. Recommendation of treatment depends on a variety of factors, including the likelihood of liver disease progression, likelihood of success, current alcohol or drug use, and potential for additive side effects. It is important to note that low rates of both eligibility and access to treatment for IFN have been observed in some cohorts of coinfected subjects [64, 65].
|
|
| |
| |
 |
|
| |
| |
|
|
| |
| |
 |
|
| |
| |
*SVR is defined as HCV RNA < 60 IU/mL 24 weeks after completion of therapy. †In RIBAVIC, comparison was genotype 1/4 versus genotype 2/3. P < 0.05 for comparison with IFN/RBV. §P < 0.001 for comparison of genotype 1 versus nongenotype 1. APRICOT--AIDS Pegasys Ribavirin International Co-infection Trial; ETVR--end-of-treatment virologic response; HCV--hepatitis C virus; HIV-1--human immunodeficiency virus type 1; IFN--interferon; NR--not reported; PEG--peginterferon; RBV--ribavirin; RNA--ribonucleic acid; SVR--sustained virologic response.
Special considerations of IFN-based treatment in HIV-positive individuals
The complexity of treating two infections simultaneously confronts the practitioner with special considerations when using IFN-based treatments. In particular, additive toxicities can be observed, especially myelosuppression and anemias. Moreover, RBV has been observed in vitro to antagonize the effects of zidovudine (AZT) and stavudine (d4T), while enhancing ddI phosphorylation. A pharmacokinetic substudy within the APRICOT trial found no significant impact of RBV on AZT or d4T phosphorylation, suggesting that an adverse interaction does not exist between these agents in vivo [66]. (note from Jules Levin: for patients taking AZT who add RBV, there have reports of increased anemia).
Importantly, ddI has been clinically associated in combination with IFN and RBV with fatal cases of lactic acidosis [67,68]. Therefore, it is strongly recommended that practitioners switch to an alternative non-ddI-containing regimen prior to the initiation of IFN and RBV-based therapy.
Transient CD4+ lymphopenia while on IFN-based therapy was repeatedly observed in many studies; however, the relative percentage of CD4 T cells tends to increase. Thus far, no increase in HIV-1 disease progression or incidence of opportunistic infections has been detected in subjects on IFN. A substudy of APRICOT among persons with detectable HIV-1 viremia indicated a modest anti-HIV-1 (0.9 log) effect of the peginterferon-containing regiments [61].
Because of a high prevalence of coexisting psychiatric disease in HIV-1-infected persons, close monitoring for worsening depression on IFN therapy is warranted, and provision of anticipatory psychiatric care advisable. Preemptive treatment with antidepressants is being studied in this population.
Liver transplantation
Liver transplantation is an option that is not yet widely available to HIV-infected individuals with end-stage liver disease. However, several centers are investigating the outcomes related to solid-organ transplantation in this population, and early reports suggest increasing feasibility. One recent published study showed no significant difference in survival of HIV-positive subjects undergoing orthotopic liver transplantation when compared with HIV-negative subjects, and further multicenter studies are ongoing to characterize their outcomes [69*]. Nevertheless, the paucity of available organs underscores the need to prevent end-stage liver disease through earlier evaluation and treatment of coinfected individuals.
Conclusions
Practitioners with experience treating either HIV-1 or HCV face particular challenges when confronted with HIV-1/HCV coinfection. The care of these patients necessitates an understanding of the natural history, treatment, and pathogenesis of both infections. Further study will elucidate the mechanisms underlying the interactions between the two viruses and continue to inform our management approach to this complex population.
References and Recommended Reading
Recently published papers of particular interest have been highlighted as:
* Of importance
** Of major importance
1.Sherman KE: Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis 2002, 34:831-837.
2.Tedaldi EM, et al.: Prevalence and characteristics of hepatitis C virus coinfection in a human immunodeficiency virus clinical trials group: the Terry Beirn Community Programs for Clinical Research on AIDS. Clin Infect Dis 2003, 36:1313-1317.
3.Sulkowski MS: Hepatitis C in the HIV-infected person. Ann Intern Med 2003, 138:197-207.
4.Brau N, et al.: Prevalence of hepatitis C and coinfection with HIV among United States veterans in the New York City metropolitan area. Am J Gastroenterol 2002, 97:2071-2078.
5.Alter MJ, et al.: The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med 1999, 341:556-562.
6.Eyster ME: Increasing hepatitis C virus RNA levels in hemophiliacs: relationship to human immunodeficiency virus infection and liver disease. Multicenter Hemophilia Cohort Study. Blood 1994, 84:1020-1023.
7.Benhamou Y, et al.: Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology 1999, 30:1054-1058.
8.Puoti M, et al.: Liver fibrosis progression is related to CD4 cell depletion in patients coinfected with hepatitis C virus and human immunodeficiency virus. J Infect Dis 2001, 183:134-137.
9.Martinez-Sierra C, et al.: Progression of chronic hepatitis C to liver fibrosis and cirrhosis in patients coinfected with hepatitis C virus and human immunodeficiency virus. Clin Infect Dis 2003, 36:491-498.
10.Graham CS, et al.: Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis 2001, 33:562-569.
11.Bica I, et al.: Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis 2001, 32:492-497.
12.Martin-Carbonero L, et al.: Incidence and predictors of severe liver fibrosis in human immunodeficiency virus-infected patients with chronic hepatitis C: a European collaborative study. Clin Infect Dis 2004, 38:128-133.
13.Sabin CA, et al.: The association between hepatitis C virus genotype and human immunodeficiency virus disease progression in a cohort of hemophilic men. J Infect Dis 1997, 175:164-168.
14.Piroth L, et al.: Does hepatitis C virus co-infection accelerate clinical and immunological evolution of HIV-infected patients? AIDS 1998, 12:381-388.
15.* Greub G, et al.: Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study. Lancet 2000, 356:1800-1805.
This large study first identified an association between HCV coinfection and HIV disease progression and found a blunting of immune recovery after initiation of HAART among HCV-coinfected persons.
16.De Luca A, et al.: Coinfection with hepatitis viruses and outcome of initial antiretroviral regimens in previously naive HIV-infected subjects. Arch Intern Med 2002, 162:2125-2132.
17.* Sulkowski MS, et al.: Hepatitis C and progression of HIV disease. JAMA 2002, 288:199-206.
This study failed to identify accelerated progression of HIV disease or impaired antiretroviral therapy-induced immune recovery among HCV-coinfected persons in a large urban US cohort.
18.Chung RT, et al.: Immune recovery is associated with persistent rise in hepatitis C virus RNA, infrequent liver test flares, and is not impaired by hepatitis C virus in co-infected subjects. AIDS 2002, 16:1915-1923.
19.Graham CS: Why should hepatitis C affect immune reconstitution in HIV-1-infected patients? Lancet 2000, 356:1865-1866.
20.Hunt PW, et al.: T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis 2003, 187:1534-1543.
21.Sulkowski MS: Hepatotoxicity associated with antiretroviral therapy in adults infected with human immunodeficiency virus and the role of hepatitis C or B virus infection. JAMA 2000, 283:74-80.
22.Saves M, et al.: Severe hepatic cytolysis: incidence and risk factors in patients treated by antiretroviral combinations. Aquitaine Cohort, France, 1996-1998. Groupe dEpidemiologie Clinique de Sida en Aquitaine (GECSA). AIDS 1999, 13:F115-F121.
23.den Brinker M, et al.: Hepatitis B and C virus co-infection and the risk for hepatotoxicity of highly active antiretroviral therapy in HIV-1 infection. AIDS 2000, 14:2895-2902.
24.Martinez E, et al.: Hepatotoxicity in HIV-1-infected patients receiving nevirapine-containing antiretroviral therapy. AIDS 2001, 15:1261-1268.
25.Monforte Ade A, et al.: Low frequency of severe hepatotoxicity and association with HCV coinfection in HIV-positive patients treated with HAART. J Acquir Immune Defic Syndr 2001, 28:114-123.
26.Nunez M, et al.: Risk factors for severe hepatic injury after introduction of highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2001, 27:426-431.
27.Sulkowski MS, et al.: Hepatotoxicity associated with nevirapine or efavirenz-containing antiretroviral therapy: role of hepatitis C and B infections. Hepatology 2002, 35:182-189.
28.Wit FW, et al.: Incidence of and risk factors for severe hepatotoxicity associated with antiretroviral combination therapy. J Infect Dis 2002, 186:23-31.
29.Johnson S: Adverse effects associated with use of nevirapine in HIV postexposure prophylaxis for 2 health care workers. JAMA 2000, 284:2722-2723.
30.Bonnet F, et al.: A cohort study of nevirapine tolerance in clinical practice: French Aquitaine Cohort, 1997-1999. Clin Infect Dis 2002, 35:1231-1237.
31.Macias J, et al.: Effect of antiretroviral drugs on liver fibrosis in HIV-infected patients with chronic hepatitis C: harmful impact of nevirapine. AIDS 2004, 18:767-774.
32.Mehta SH, et al.: The effect of HAART and HCV infection on the development of hyperglycemia among HIV-infected persons. J Acquir Immune Defic Syndr 2003, 33:577-584.
33.Pol S: HIV infection and hepatic enzyme abnormalities: intricacies of the pathogenic mechanisms. Clin Infect Dis 2004, 38(suppl 2):S65-S72.
34.Sterling RK, et al.: Impact of highly active antiretroviral therapy on the spectrum of liver disease in HCV-HIV coinfection. Clin Gastroenterol Hepatol 2004, 2:432-439.
35.Benhamou Y, et al.: Factors affecting liver fibrosis in human immunodeficiency virus-and hepatitis C virus-coinfected patients: impact of protease inhibitor therapy. Hepatology 2001, 34:283-287.
36.* Cooper CL: Review of the effect of highly active antiretroviral therapy on hepatitis C virus (HCV) RNA levels in human immunodeficiency virus and HCV coinfection. Clin Infect Dis 2002, 35:873-879.
Review of several conflicting studies regarding the effects of HAART on HCV levels. Although the authors found much heterogeneity between studies, overall an early increase in HCV viral loads appears to be followed by a later slight decrease.
37.Zylberberg H, et al.: Rapidly evolving hepatitis C virus-related cirrhosis in a human immunodeficiency virus-infected patient receiving triple antiretroviral therapy. Clin Infect Dis 1998, 27:1255-1258.
38.Vento S, et al.: Enhancement of hepatitis C virus replication and liver damage in HIV-coinfected patients on antiretroviral combination therapy. AIDS 1998, 12:116-117.
39.Fialaire P, et al.: Sustained disappearance of hepatitis C viremia in patients receiving protease inhibitor treatment for human immunodeficiency virus infection. J Infect Dis 1999, 180:574-575.
40.Yokozaki S, et al.: Immunologic dynamics in hemophiliac patients infected with hepatitis C virus and human immunodeficiency virus: influence of antiretroviral therapy. Blood 2000, 96:4293-4299.
41.Perez-Olmeda M: Hepatitis C viraemia in HIV-HCV co-infected patients having immune restoration with highly active antiretroviral therapy. AIDS 2000, 14:212.
42.Ranieri R: Hepatitis C viremia persistently suppressed by HAART. Clin Infect Dis 2003, 36:1086-1087.
43.Bare P, et al.: HCV recovery from peripheral blood mononuclear cell culture supernatants derived from HCV-HIV co-infected haemophilic patients with undetectable HCV viraemia. Haemophilia 2003, 9:598-604.
44.* Qurishi N, et al.: Effect of antiretroviral therapy on liver-related mortality in patients with HIV and hepatitis C virus coinfection. Lancet 2003, 362:1708-1713.
This analysis of 285 coinfected subjects in Germany shows a positive effect of HAART on both overall and HCV-related mortality.
45.Rosenberg PM, et al.: Rapidly progressive fibrosing cholestatic hepatitis--hepatitis C virus in HIV coinfection. Am J Gastroenterol 2002, 97:478-483.
46.Einav S: Immunopathogenesis of hepatitis C virus in the immunosuppressed host. Transpl Infect Dis 2002, 4:85-92.
47.Racanelli V: Hepatitis C virus infection: when silence is deception. Trends Immunol 2003, 24:456-464.
48.Masur H: Guidelines for preventing opportunistic infections among HIV-infected persons--2002. Recommendations of the U.S. Public Health Service and the Infectious Diseases Society of America. Ann Intern Med 2002, 137(5 Pt 2):435-478.
49.Takaki A, et al.: Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat Med 2000, 6:578-582.
50.Palella FJ Jr, et al.: Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 1998, 338:853-860.
51.Tedaldi EM, et al.: Influence of coinfection with hepatitis C virus on morbidity and mortality due to human immunodeficiency virus infection in the era of highly active antiretroviral therapy. Clin Infect Dis 2003, 36:363-367.
52.Puoti M, et al.: Severe hepatotoxicity during combination antiretroviral treatment: incidence, liver histology, and outcome. J Acquir Immune Defic Syndr 2003, 32:259-267.
53.Uberti-Foppa C, et al.: Pretreatment of chronic active hepatitis C in patients coinfected with HIV and hepatitis C virus reduces the hepatotoxicity associated with subsequent antiretroviral therapy. J Acquir Immune Defic Syndr 2003, 33:146-152.
54.Sulkowski MS: Drug-induced liver injury associated with antiretroviral therapy that includes HIV-1 protease inhibitors. Clin Infect Dis 2004, 38(suppl 2):S90-S97.
55.Zylberberg H, et al.: Safety and efficacy of interferon-ribavirin combination therapy in HCV-HIV coinfected subjects: an early report. Gut 2000, 47:694-697.
56.Nasti G, et al.: Chronic hepatitis C in HIV infection: feasibility and sustained efficacy of therapy with interferon alfa-2b and tribavirin. AIDS 2001, 15:1783-1787.
57.Landau A, et al.: Long-term efficacy of combination therapy with interferon-alpha 2b and ribavirin for severe chronic hepatitis C in HIV-infected patients. AIDS 2001, 15:2149-2155.
58.Sauleda S, et al.: Interferon and ribavirin combination therapy for chronic hepatitis C in human immunodeficiency virus-infected patients with congenital coagulation disorders. Hepatology 2001, 34:1035-1040.
59.Brau N, et al.: Treatment of chronic hepatitis C in HIV/HCV-coinfection with interferon alpha-2b+ full-course vs. 16-week delayed ribavirin. Hepatology 2004, 39:989-998.
60.Chung R, et al.: A randomized, controlled trial of PEG-interferon-alfa-2a plus ribavirin vs interferon-alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-co-infected persons: follow-up results of ACTG A5071. Paper presented at the 11th Conference on Retroviruses and Opportunistic Infections. San Francisco, CA; February 8-11, 2004.
61.Torriani FJ, et al.: Final results of APRICOT: a randomized, partially blinded, international trial evaluating peginterferon-alfa-2a + ribavirin vs. interferon-alfa-2a + ribavirin in the treatment of HCV in HIV/HCV co-infection. Paper presented at the 11th Conference on Retroviruses and Opportunistic Infections. San Francisco, CA; February 8-11, 2004.
62.Perronne C, et al.: Final results of ANRS HC02-RIBAVIC: A randomized controlled trial of pegylated-interferon-alfa-2b plus ribavirin vs. interferon-alfa-2b plus ribavirin for the initial treatment of chronic hepatitis C in HIV co-infected patients. Paper presented at the 11th Conference on Retroviruses and Opportunistic Infections. San Francisco, CA; February 8-11, 2004.
63.Afdhal NH, et al.: Epoetin alfa maintains ribavirin dose in HCV-infected patients: a prospective, double-blind, randomized controlled study. Gastroenterology 2004, 126:1302-1311.
64.Fleming CA, et al.: Hepatitis C virus and human immunodeficiency virus coinfection in an urban population: low eligibility for interferon treatment. Clin Infect Dis 2003, 36:97-100.
65.Hall CS, et al.: Hepatitis C virus infection in San Francisco"s HIV-infected urban poor. J Gen Intern Med 2004, 19:357-365.
66.Gries JM, et al.: Effect of ribavirin on intracellular and plasma pharmacokinetics of nucleoside reverse transcriptase inhibitors in patients with HCV/HIV co-infection: final results of a randomized clinical study. Paper presented at the 11th Conference on Retroviruses and Opportunistic Infections. San Francisco, CA; February 8-11, 2004.
67.Lafeuillade A: Increased mitochondrial toxicity with ribavirin in HIV/HCV coinfection. Lancet 2001, 357:280-281.
68.Moreno A, et al.: High rate of didanosine-related mitochondrial toxicity in HIV/HCV-coinfected patients receiving ribavirin. Antivir Ther 2004, 9:133-138.
69.* Ragni MV, et al.: Survival of human immunodeficiency virus-infected liver transplant recipients. J Infect Dis 2003, 188:1412-1420.
This study describes the short-term clinical outcome of persons undergoing liver transplantation for end-stage liver disease who are HIV positive, demonstrating comparable survival to HIV-negative persons with similar indications.
|
|
| |
| |
|
|
|