| |
Why HCV Therapy Is Not Utilized in Coinfection
|
| |
| |
This is why we need support programs for coinfected patients who need HCV therapy.
JAIDS Journal of Acquired Immune Deficiency Syndromes: Volume 38(2) 1 February 2005 pp 238-240
"...low utility & applicability of HCV therapy would allow HCV to exert its full impact on morbidity& mortality in HCV/HIV coinfected patients...less than one third of the HIV/HCV-coinfected patients at our institution were eligible for HCV treatment and less than 10% embarked on Peg/IN plus RBV therapy (see discussion near end of report for reasons listed for not treating coinfected patients). These findings are in agreement with those of other recently published reports... Efforts are needed to improve applicability of HCV therapy in these difficult-to-treat patients who are often excluded in clinical trials..."
Chronic Hepatitis C in HIV-Infected Patients: Low Eligibility and Applicability of Therapy With Pegylated Interferon-α Plus Ribavirin
Rauch, Andri MD*; Egger, Martin MD*; Reichen, Jürg MD†; Furrer, Hansjakob MD*; The Swiss HIV Cohort Study
*Division of Infectious Diseases, University Hospital, Inselspital Berne, Berne, Switzerland; and †Institute of Clinical Pharmacology, University Hospital, Inselspital Berne, Berne, Switzerland
Supported by a research grant by Essex Chemie AG, Switzerland, and performed in the framework of the Swiss HIV Cohort Study, supported by the Swiss National Science Foundation (grant 3347-069366).
To the Editor:
Chronic hepatitis C virus (HCV) infection is currently a leading cause of morbidity and mortality in HIV-infected patients in developed countries.1-3 The recommended therapeutic strategy is to eradicate HCV infection by using combination therapy with pegylated interferon-α (pegIFN) plus ribavirin (RBV).4,5 Randomized clinical trials using pegIFN plus RBV in HIV/HCV-coinfected patients suggest a sustained virologic response rate from 15% (HCV genotype 1 or 4) up to 70% (HCV genotype 2 or 3).6-8 Results of clinical trials may not be representative for the entire HIV/HCV-coinfected population, however. In some cohorts of HIV/HCV-coinfected patients, less than 10% of coinfected individuals embarked on pegIFN plus RBV therapy.9-11 Low applicability of HCV therapy would allow HCV infection to exert nearly its full impact on morbidity and mortality in HIV/HCV-coinfected patients. Therefore, we prospectively evaluated the eligibility and applicability of pegIFN plus RBV therapy in a representative cohort of HIV/HCV-coinfected individuals.
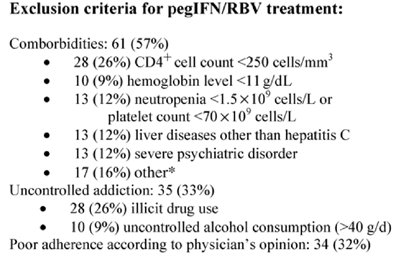
All patients evaluated for this prospective single-center cohort study received primary HIV care at the outpatient clinic of the University Hospital Berne, Berne, Switzerland. Patients had to be enrolled in the Swiss HIV Cohort Study (SHCS) to be eligible for analysis. In the SHCS, information about demographics, HIV-associated diseases, medications, and laboratory parameters is collected according to standardized criteria at registration and at follow-up visits at intervals of 6 months (available at: www.shcs.ch ). We enrolled all SHCS participants seropositive for HCV who had at least 1 follow-up visit from March 2003 to September 2003. Inclusion and exclusion criteria were implemented according to commonly used treatment protocols for pegIFN plus RBV treatment in HIV/HCV-coinfected patients.4-8,12,13 Exclusion criteria included a CD4 cell count <250 cells/mm3, anemia (hemoglobin level <11g/dL), cytopenia (neutrophils <1.5 × 109 cells/L or a platelet count <70 × 109 cells/L), liver diseases other than hepatitis C, decompensated liver disease, significant comorbidities (eg, psychiatric disorders, seizures, cardiopulmonary disease, immunologically mediated diseases), uncontrolled addiction (illicit drug abuse or alcohol consumption >40g/d), and pregnancy. Poor adherence to prescribed drugs (according to the treating physicians) was also considered an exclusion criterion. Normal liver function test results were not an exclusion criterion. Exclusion criteria were assessed consecutively for all HIV/HCV-coinfected patients by using a standardized questionnaire.
A liver biopsy was suggested to patients eligible for treatment. Every eligible patient was discussed by a panel of infectious disease specialists and hepatologists. In eligible patients who chose to postpone treatment, the applicability of HCV treatment was reassessed systematically at least once more between October 2003 and February 2004. The local ethical committee approved the study, and written informed consent was obtained before starting therapy.
Eligibility was defined as the proportion of patients lacking any exclusion criteria, whereas applicability was defined as the proportion of patients starting pegIFN plus RBV treatment during the observation period. STATA (version 8.0) software was used for statistical analysis.
Of 459 HIV-infected patients receiving primary HIV care from March 2003 to September 2004 in our HIV outpatient clinic, 135 (29%) were seropositive for HCV, and 107 (79%) of those had detectable HCV RNA. Of these 107 chronically infected patients, 68 (64%) were male and the median age was 39 years (interquartile range [IQR]: 36-43 years). The median CD4 cell count was 369 cells/mm3 (IQR: 236-499 cells/mm3). Eighty-four (79%) patients were on highly active antiretroviral therapy, and 66% of these had undetectable HIV RNA. Fifty-eight (54%) patients were in a drug substitution program, and 28 (26%) reported the current use of intravenous drugs. Seventy percent of the evaluated patients had HCV genotype 1 or 4, whereas HCV genotype 2 or 3 was found in 30%.
|
|
| |
| |
 |
|
| |
| |
Eighty-two (77%) of the 107 HIV/HCV-coinfected patients were not eligible for pegIFN plus RBV treatment (Fig. 1). Most of the ineligible patients had multiple exclusion criteria: 73% had more than 1, and 33% had more than 3. The most frequent reasons for exclusion were low CD4 cell counts, cytopenia, hepatic disorders other than hepatitis C, psychiatric illnesses, uncontrolled addiction, and poor adherence. Only 10% of female patients but 31% of male patients were eligible (P < 0.05). There were no further statistically significant differences regarding demographic characteristics between eligible and ineligible patients. Of the 25 treatment-eligible patients, 16 (64%) decided not to proceed, mainly based on fear of side effects or because they considered treatment procedures to be too time-consuming. Four patients with HCV genotype 1 decided to postpone treatment because the liver biopsy showed only mild fibrosis. Nine (8%) of 107 HIV/HCV-coinfected patients started combination treatment with pegIFN plus RBV.
In conclusion, less than one third of the HIV/HCV-coinfected patients at our institution were eligible for HCV treatment and less than 10% embarked on Peg/IN plus RBV therapy. These findings are in agreement with those of other recently published reports.9-11 Most (79%) of the ineligible patients were on combination antiretroviral therapy, and 66% of these individuals had undetectable HIV RNA, indicating that many of these patients were able to adhere reasonably well to prescribed medication. The finding that even these patients could not be treated for chronic hepatitis C illustrates the significant barriers for treatment with pegIFN plus RBV using inclusion criteria derived from clinical trials. In our region, most patients seeking primary HIV care are treated at our center and are enrolled in the SHCS. The results of our study may be representative for a general population of HIV/HCV-coinfected patients. Nevertheless, our study may overestimate the proportion of eligible patients, because the subgroup of drug addicts not seeking primary HIV care has not been evaluated. Seven (78%) of the treated patients were individuals with genotype 3, and only 2 patients with genotype 1 were treated. This reflects the reluctance of physicians and patients to start pegIFN plus RBV therapy facing the low response rate of combination therapy in the latter patients.6-8 In our study, the proportion of female patients was significantly lower in the group of treatment-eligible individuals; however, the numbers were small, and the significance of this finding remains unclear.
In our setting, pegIFN plus RBV therapy is not likely to have a major impact on the course of HCV infection in coinfected patients on a population level regardless of the efficacy of pegIFN plus RBV treatment protocols. Efforts are needed to improve applicability of HCV therapy in these difficult-to-treat patients who are often excluded in clinical trials.
Andri Rauch, MD*
Martin Egger, MD*
Jürg Reichen, MD†
Hansjakob Furrer, MD* and the Swiss HIV Cohort Study
*Division of Infectious Diseases, University Hospital, Inselspital Berne, Berne, Switzerland; †Institute of Clinical Pharmacology, University Hospital, Inselspital Berne, Berne, Switzerland
REFERENCES
1. Bica I, McGovern B, Dhar R, et al. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32:492-497.
2. Greub G, Ledergerber B, Battegay M, et al. Clinical progression, survival, and immune recovery during antiretroviral therapy in patients with HIV-1 and hepatitis C virus coinfection: the Swiss HIV Cohort Study. Lancet. 2000;356:1800-1805.
3. Monga HK, Rodriguez-Barradas MC, Breaux K, et al. Hepatitis C virus infection-related morbidity and mortality among patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;33:240-247.
4. Seeff LB, Hoofnagle JH. National Institutes of Health Consensus Development Conference: management of hepatitis C: 2002. Hepatology. 2002;36(Suppl):S1-S2.
5. Soriano V, Puoti M, Sulkowski M, et al. Care of patients with hepatitis C and HIV co-infection. AIDS. 2004;18:1-12.
6. Chung RT, Andersen J, Volberding P, et al. Peginterferon alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351:451-459.
7. Perrone C, Carrat F, Bani-Sadr F, et al. Final results of ANRS HCO2-RIBAVIC: a randomized controlled trial of pegylated interferon-alfa-2b plus ribavirin vs interferon-alfa-2b plus ribavirin for the initial treatment of chronic hepatitis C in HIV co-infected patients [abstract 2004. A3295]. Presented at: XV International AIDS Conference; 2004; Bangkok.
8. Torriani FJ, Rodriguez-Torres M, Rockstroh JK, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438-450.
9. Fleming CA, Craven DE, Thornton D, et al. Hepatitis C virus and human immunodeficiency virus coinfection in an urban population: low eligibility for interferon treatment. Clin Infect Dis. 2003;36:97-100.
10. Fultz SL, Justice AC, Butt AA, et al. Testing, referral, and treatment patterns for hepatitis C virus coinfection in a cohort of veterans with human immunodeficiency virus infection. Clin Infect Dis. 2003;36:1039-1046.
11. Hall CS, Charlebois ED, Hahn JA, et al. Hepatitis C virus infection in San Francisco's HIV-infected urban poor. J Gen Intern Med. 2004;19:357-365.
12. Moreno L, Quereda C, Moreno A, et al. Pegylated interferon alpha2b plus ribavirin for the treatment of chronic hepatitis C in HIV-infected patients. AIDS. 2004;18:67-73.
13. Perez-Olmeda M, Nunez M, Romero M, et al. Pegylated IFN-alpha2b plus ribavirin as therapy for chronic hepatitis C in HIV-infected patients. AIDS. 2003;17:1023-1028.
APPENDIX
The members of the SHCS are M. Battegay, E. Bernasconi, J. Boeni, H. Bucher, Ph. Bürgisser, S. Cattacin, M. Cavassini, R. Dubs, M. Egger, L. Elzi, P. Erb, K. Fantelli, M. Fischer, M. Flepp, A. Fontana, P. Francioli (President of the SHCS, Centre Hospitalier Universitaire Vaudois, CH-1011 Lausanne), H. Furrer (Chairman of the Clinical and Laboratory Committee), M. Gorgievski, H. Günthard, B. Hirschel, L. Kaiser, C. Kind, Th. Klimkait, B. Ledergerber, U. Lauper, M. Opravil, F. Paccaud, G. Pantaleo, L. Perrin, J.-C. Piffaretti, M. Rickenbach (Head of Data Center), C. Rudin (Chairman of the Mother and Child Substudy), P. Schmid, J. Schüpbach, R. Speck, A. Telenti, A. Trkola, P. Vernazza (Chairman of the Scientific Board), R. Weber, and S. Yerly.
|
|
| |
| |
|
|
|