| |
Pegasys + Methadone PK
|
| |
| |
"Peginterferon alfa-2a does not alter the pharmacokinetics of methadone in patients with chronic hepatitis C undergoing methadone maintenance therapy"
Clinical Pharmacology & Therapeutics
March 2005
The objective of this study was to quantitatively evaluate the effects of peginterferon alfa-2a (Pegasys) on the pharmacokinetics of methadone in patients with chronic hepatitis C who were receiving a stable methadone maintenance regimen. The pharmacokinetics and pharmacodynamics of peginterferon alfa-2a were also quantified in these individuals.
Injection drug use is the most common mode of acquisition of new hepatitis C virus (HCV) infections in the United States. Injection drug users (IDUs) are at high risk of HCV infection because of the efficiency with which the virus is parenterally transmitted. Indeed, within 1 year of needle use, approximately 77% of intravenous drug users become infected with HCV; in those who have used injectable drugs for 10 years or more, the prevalence of HCV exposure is as high as 94%.
Until recently, methadone treatment has been a strict exclusion criterion in clinical trials, and as a result, patients with hepatitis C who are receiving methadone maintenance therapy have been identified as an understudied population. Thus this study was designed to address this critical problem of chronic hepatitis C in methadone recipients.
Comprehensive strategies for the management of HCV infection must encompass IDUs. Methadone maintenance programs are effective in diminishing opioid drug use and decreasing other high-risk behaviors; accordingly, such drug treatment programs may serve as an important link to provide IDUs with access to treatment for chronic hepatitis C.
STUDY REPORTS:
concurrent treatment with methadone is not a contraindication to therapy with peginterferon alfa-2a for chronic hepatitis C
".....preliminary, these encouraging results suggest that patients with a recent history of injection drug use, particularly those undergoing stable methadone maintenance therapy, can be successfully treated for hepatitis C with interferon-based regimens....
.....recent studies have suggested that sustained viral response rates in patients receiving methadone maintenance therapy are similar to those observed in other HCV-infected patient populations.....
.......results suggest that patients with a recent history of injection drug use, particularly those undergoing stable methadone maintenance therapy, can be successfully treated for hepatitis C with interferon-based regimens....
.......researchers report- that methadone dose was increased in some patients during treatment with conventional interferon alfa...but these increases in methadone dose generally represented the use of methadone to treat side effects of conventional interferon rather than a response to clinical evidence of a pharmacokinetic interaction"
KEY STUDY FINDINGS:
1. Peginterferon alfa-2a does not appreciably alter the pharmacokinetics of methadone. Authors stated: "it remains to be determined whether ribavirin has any influence on the pharmacokinetics of methadone". I don't recall any studies of ribavirin-methadone PK although i don't suspect there would be a significant interaction.
2. Methadone levels after 4 doses of peginterferon alfa-2a increased by 10% to 15% when compared with baseline.
3. The viral response to peginterferon alfa-2a was similar in patients receiving ongoing methadone maintenance therapy. The decrease in HCV RNA levels during the first 4 weeks of treatment with peginterferon alfa-2a was typical of that seen in chronic hepatitis C patients who are not receiving methadone maintenance therapy, and one half of the patients had a substantial reduction in HCV RNA after only 4 doses of peginterferon alfa-2a.
4. authors say: "a priori adjustment of either the methadone or the peginterferon alfa-2a dose is not recommended in patients receiving stable methadone maintenance therapy who undergo treatment for chronic hepatitis C with peginterferon alfa-2a-containing regimens"
5. Intersubject variabilities at baseline and during week 4 were 36% and 34%, respectively, for Cmax and 28% and 33%, respectively, for AUC0-24. Of the patients, 8 of 22 (36%) and 16 of 21 (76%) had higher individual Cmax and AUC0-24 values, respectively, during week 4 than at baseline.
6. see safety profile below. There were no serious adverse events, and no patients withdrew prematurely because of adverse events or laboratory abnormalities. In addition, no subject had clinical signs or symptoms of intoxication or withdrawal related to methadone.
Authors: Mark Sulkowski, MD *, Teresa Wright, MD, Stephen Rossi, PharmD
Sanjeev Arora, MD, Matthew Lamb, PharmD, Ka Wang, PhD
Jean-Michel Gries, PhD, Sreeni Yalamanchili, PharmD
Viral Hepatitis Center, Johns Hopkins University School of Medicine, Baltimore; Gastrointestinal Section, Veterans Affairs Medical Center, University of California, San Francisco, San Francisco; Science Center, University of New Mexico, Albuquerque; Roche, Nutley; and Amgen, Inc, Thousand Oaks
The study was sponsored by Roche, Nutley, NJ.
ABSTRACT
Objective: Our objective was to quantify the pharmacokinetics of methadone and the pharmacokinetics and pharmacodynamics of peginterferon alfa-2a (40 kd) in patients with chronic hepatitis C undergoing methadone maintenance therapy.
Methods: Adults with chronic hepatitis C who had been receiving a consistent methadone maintenance regimen for at least 3 months were eligible for this open-label, multicenter, nonrandomized drug interaction study. All patients received 180 EÊg subcutaneous peginterferon alfa-2a once weekly for 4 weeks and continued their methadone regimen. Serial blood samples were collected at baseline and immediately before and for up to 168 hours after study drug administration for the purposes of quantifying methadone and peginterferon alfa-2a serum concentrations, measuring serum 2Aa,5Aa-oligoadenylate synthetase activity, and determining hepatitis C virus ribonucleic acid levels.
Results: Twenty-four patients were enrolled. Methadone exposure, as measured by maximum serum concentration (Cmax) and area under the concentration-time curve (AUC) normalized to a 100-mg/d dose, after 4 doses of peginterferon alfa-2a increased by 10% to 15% when compared with baseline. The week 4/baseline ratio of the mean Cmax was 1.11 (90% confidence interval [CI], 1.02-1.22), and for AUC from time 0 to 24 hours, the week 4/baseline ratio was 1.15 (90% CI, 1.08-1.23). The mean accumulation ratios (week 4/first dose) for Cmax and AUC from time 0 to 168 hours of peginterferon alfa-2a were 2.1 and 2.3, respectively.
Conclusions: Peginterferon alfa-2a does not appreciably alter the pharmacokinetics of methadone.
RESULTS
A total of 24 patients were enrolled in the study, 22 of whom received 4 doses of peginterferon alfa-2a and completed the pharmacokinetic portion of the study. Data from 2 patients who received only 3 doses of peginterferon alfa-2a were excluded from the pharmacokinetic analyses. In addition, pharmacokinetic data for peginterferon alfa-2a from 2 further patients were excluded because of analytic artifacts. All patients were included in the safety analysis. The baseline characteristics of the patients are presented in Table I. The mean methadone dose at baseline was 88 mg/d.
Table I. Baseline characteristics of 24 patients enrolled in study
| Characteristic |
No. |
| Male/female |
15/9 |
| Race (white/black/other) |
13/6/5 |
| Age (y) (median and range) |
49.5(33-61) |
| Weight (kg) (median and range) |
78.5(52-129) |
| Body mass index (kg/m2) (medianand range) |
26.9(19.1-48.9) |
| Methadone dose (mg/d) (medianand range) |
95(30-150) |
Pharmacokinetic results
Influence of peginterferon alfa-2a on pharmacokinetics of methadone
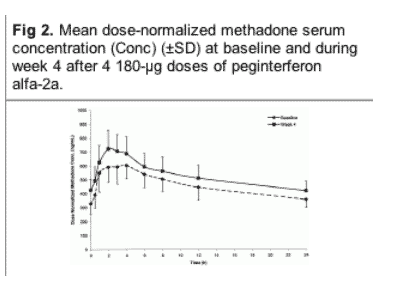
Methadone exposure after 4 doses of peginterferon alfa-2a increased by 10% to 15% when compared with baseline. However, the 90% CIs for the ratio of the mean Cmax and AUC0-24 at baseline and during week 4 were 1.02 to 1.22 and 1.08 to 1.23, respectively, which fell within the generally accepted equivalence interval of 0.80 to 1.25, indicating no significant effect of peginterferon alfa-2a on the pharmacokinetics of methadone.
Intersubject variabilities at baseline and during week 4 were 36% and 34%, respectively, for Cmax and 28% and 33%, respectively, for AUC0-24. Of the patients, 8 of 22 (36%) and 16 of 21 (76%) had higher individual Cmax and AUC0-24 values, respectively, during week 4 than at baseline.
The mean 24-hour serum concentration profile for methadone (normalized to a 100-mg dose) at baseline and during week 4 is presented in Fig 2. Methadone concentrations were higher at each time point during the 24-hour sampling period.
|
|
 |
| |
Influence of methadone on pharmacokinetics of peginterferon alfa-2a
The mean accumulation ratios (week 4/first dose) for Cmax and AUC0-168 were 2.1 (90% CI, 1.62-2.58) and 2.3 (90% CI, 1.82-2.78), respectively. Values for peginterferon alfa-2a during week 1 and week 4 reveal a general increase during the study.
| |
First Dose |
Week 4 |
| CMax |
14.4±5.4 |
26.0±10.9 |
| AUC last |
1769±669 |
3459±1451 |
| AUC 0-168: |
1788±700 |
3515±1463 |
Accumulation ratio Cmax: NA 2.1
Accumulation ratio AUC 0-168: NA 2.3
Pharmacodynamic results
The induction of 2Aa,5Aa-OAS activity in methadone recipients was similar to that previously reported in subjects not receiving methadone maintenance therapy.
Safety
A total of 123 adverse events were reported by 20 patients (83%). The most common adverse events were those typically associated with conventional interferon or pegylated interferon administration (ie, flulike symptoms). Of the patients, 4 (17%) had a neutrophil count of less than 0.75 ~ 109/L at some point during the trial but no patient had a neutrophil count of less than 0.50 ~ 109/L. Of the patients, 2 (8%) had a platelet count of less than 50 ~ 109/L at some point during the trial but no patient had a platelet count of less than 20 ~ 109/L. There were no serious adverse events, and no patients withdrew prematurely because of adverse events or laboratory abnormalities. In addition, no subject had clinical signs or symptoms of intoxication or withdrawal related to methadone.
AUTHOR DISCUSSION
The results of our study demonstrate that peginterferon alfa-2a does not influence the pharmacokinetics of methadone to a clinically significant extent in patients receiving ongoing methadone maintenance therapy. Baseline pharmacokinetic and pharmacodynamic data for peginterferon alfa-2a in the absence of methadone administration were not available for the patients enrolled in our study; however, the pharmacokinetic profile of peginterferon alfa-2a in these individuals was generally similar to that observed previously in healthy volunteers and patients with chronic hepatitis C.29-32
The pharmacokinetic values for racemic methadone reported in our study (normalized to 100 mg) compare well with those reported previously in opiate users (normalized to 10 mg14 or 70 mg16). It must be noted that we did not evaluate the pharmacokinetic properties of the active R-isomer of methadone, so it is not possible to comment on the impact of peginterferon alfa-2a on the pharmacokinetics of this entity. We also did not monitor Eo1-acid glycoprotein levels, which have an influence on the plasma pharmacokinetics of the drug.15
As anticipated, the mean serum concentrations of peginterferon alfa-2a were higher during week 4 than during week 1. This agent has a long elimination half-life and, as a result, does not reach steady-state levels until 6 to 8 weeks after the initiation of weekly administration. The accumulation ratios (steady-state concentration/maximum concentration after 1 dose) reported previously have been approximately 2 to 3.29,32 Thus our finding of an accumulation ratio of approximately 2 after 4 weeks of weekly dosing is in line with our expectations.
The biologic response to peginterferon alfa-2a, as assessed by 2Aa 5Aa-OAS activity, was similar in patients receiving ongoing methadone maintenance therapy in our study and in healthy subjects enrolled in previous studies.31,33 The decrease in HCV RNA levels during the first 4 weeks of treatment with peginterferon alfa-2a was typical of that seen in chronic hepatitis C patients who are not receiving methadone maintenance therapy, and one half of the patients had a substantial reduction in HCV RNA after only 4 doses of peginterferon alfa-2a.34
Peginterferon alfa-2a monotherapy was well tolerated during methadone maintenance therapy in patients with chronic hepatitis C, and no serious adverse events were observed. The spectrum and frequency of adverse events were similar to those reported in patients with chronic hepatitis C who were not receiving methadone in a phase III trial,35 although it must be acknowledged that the patients in our trial were monitored for only 4 weeks.
There are several limitations to our study. As noted, we evaluated patients during an abbreviated course of treatment with peginterferon alfa-2a monotherapy rather than for the full 48-week duration recommended for the treatment of chronic hepatitis C at the time the study was planned. However, the 4-week treatment period was sufficient to confirm whether peginterferon alfa-2a interfered with the pharmacokinetics of methadone and to determine whether the pharmacodynamics of peginterferon alfa-2a was impaired by methadone. Thus, although the most important therapeutic end point in patients with chronic hepatitis C is the eradication of HCV infection, our study was not designed with sufficient statistical power to examine sustained virologic response rates. In addition, it must be noted that therapy for chronic hepatitis C has continued to evolve since our study was conceived, and the current treatment of choice is the combination of pegylated interferon plus ribavirin.7 Thus it remains to be determined whether ribavirin has any influence on the pharmacokinetics of methadone.
These limitations notwithstanding, our data provide important information to clinicians treating the relatively large population of HCV-infected persons receiving methadone maintenance therapy worldwide. Despite the size of the population, patients with chronic hepatitis C who are receiving methadone maintenance therapy have been understudied, a problem that was recognized by the US National Institutes of Health Consensus Panel in their 2002 recommendations for the management of chronic hepatitis C.7 However, there is increasing interest in the treatment of hepatitis C in methadone recipients, and recent studies have suggested that sustained viral response rates in patients receiving methadone maintenance therapy are similar to those observed in other HCV-infected patient populations. For example, among patients included in a large, retrospective, matched cohort study, Van Thiel et al36 reported that sustained virologic response rates were similar in patients receiving methadone maintenance therapy and in patients without a history of intravenous drug use after treatment with daily conventional interferon (33% and 37%, respectively). Interestingly, the researchers observed that the methadone dose was increased in some patients during treatment with conventional interferon alfa. However, consistent with our findings, the authors reported that these increases in methadone dose generally represented the use of methadone to treat side effects of conventional interferon rather than a response to clinical evidence of a pharmacokinetic interaction. Similarly, in an interim analysis of 50 methadone maintenance patients treated with conventional interferon plus ribavirin, Sylvestre37 reported that the end-of-treatment response rate was similar to that of patients without a history of injection drug use. It must be noted that we did not observe a similar increase in methadone doses during our study. A smaller, prospective, open-label German study has also reported success in treating intravenous drug users enrolled in a detoxification program with conventional interferon-based therapy.38 Although preliminary, these encouraging results suggest that patients with a recent history of injection drug use, particularly those undergoing stable methadone maintenance therapy, can be successfully treated for hepatitis C with interferon-based regimens.
In conclusion, our data indicate that peginterferon alfa-2a does not appreciably alter the pharmacokinetics of methadone. Thus concurrent treatment with methadone is not a contraindication to therapy with peginterferon alfa-2a for chronic hepatitis C. Although the decision to treat a patient who is using illicit drugs should be made by the patient after consulting with his or her physician, this decision need not be influenced by the use of methadone maintenance therapy, and a priori adjustment of either the methadone or the peginterferon alfa-2a dose is not recommended in patients receiving stable methadone maintenance therapy who undergo treatment for chronic hepatitis C with peginterferon alfa-2a-containing regimens.
INTRODUCTION
The combination of pegylated interferon plus ribavirin, the treatment of choice in patients with chronic hepatitis C,7 produces sustained virologic responses in 52% to 63% of patients with HCV monoinfection and 40% of patients with HCV/human immunodeficiency virus coinfection.8-12 Until recently, methadone treatment has been a strict exclusion criterion in clinical trials, and as a result, patients with hepatitis C who are receiving methadone maintenance therapy have been identified as an understudied population.13 Thus this study was designed to address this critical problem of chronic hepatitis C in methadone recipients.
Because of the magnitude of the epidemic of hepatitis C in IDUs, it is important to understand the clinical implications of the concomitant use of opiates (ie, methadone) and anti-HCV therapies. Moreover, potential pharmacokinetic interactions between therapies for hepatitis C and opiate agonists need to be investigated to establish the safety of concurrent HCV and methadone treatment.6
Methadone is a racemic mixture of which the R-enantiomer is the active moiety.14-17 There is marked interindividual variability in the distribution and clearance of the drug that is derived from variability in the individual serum concentrations of Eo1-acid glycoprotein and cytochrome P450 (CYP) 3A4 activity.14,16,17 The stereoselective metabolism of methadone is mediated by CYP3A4, CYP2C8, and CYP2D6, and data obtained in vitro and in vivo demonstrate that there is significant potential for drug interactions with inhibitors of these CYP isozymes.18,19
Conventional interferon alfa has been shown to inhibit various CYP isozymes including CYP3A4 and CYP2D6.20-23 Moreover, some patients with chronic hepatitis C have antibodies directed at CYP2D6,24,25 and a study conducted during the first month of treatment with interferon alfa revealed an increase in the activity of CYP3A4 and CYP2D6 in patients with chronic hepatitis C.26 It is, therefore, important to assess the potential for clinically significant CYP-mediated interactions in drugs that may be used by patients with chronic hepatitis C who are candidates for interferon-based therapy.
Methadone has been shown to have "anti-interferon" properties in mice.27 Whether the reduction in serum levels was related to decreased production or increased clearance of endogenous interferon is another unresolved but relevant question with potential implications for the treatment of chronic hepatitis C in patients undergoing methadone maintenance therapy.
|
|
| |
| |
|
|
|