| |
Sensitive HCV RNA Test: use at week 24
|
| |
| |
Letter to the Editor
"Highly sensitive hepatitis C virus RNA detection assays for decision of treatment (dis)continuation in patients with chronic hepatitis C"
Hepatology
April 2005
Ulrike Mihm, Wolf-Peter Hofmann, Bernd Kronenberger, Michael von Wagner, Stefan Zeuzem, Christoph Sarrazine
Klinik fr Innere Medizin II, Universitatsklinikum des Saarlandes, Kirrberger Stra§e, 66421 Homburg/Saar, Germany
"...according to current recommendations early discontinuation of virologic non-responders at week 12 of antiviral therapy is based on the 2 log decline/30,000IU/ml cut-off rule...
..However, on the basis of the 12 week stopping rule only 50-60% of patients without SVR are discontinued from therapy 32.
...Thus, the week 24 stopping rule is needed for discontinuation of further patients with lack of SVR...
...Current recommendations for discontinuation at week 24 are based on studies with qualitative HCV RNA detection assays with lower detection limits of 30-50IU/ml...
...In the present study, by the use of a highly sensitive detection assay with a lower detection limit of 5-10IU/ml, HCV RNA was detectable at week 24 in 6 additional patients compared to RT-PCR testing...
...Detection of HCV RNA by the highly sensitive assay at week 24 was associated in all patients with a lack of SVR following a 48 week course of (pegylated) interferon-¿/ribavirin combination therapy...
...Thus, our data suggest, that in patients with an incomplete response at week 12 a highly sensitive assay may be safely used at week 24 for the second decision whether or not to stop antiviral therapy without risking treatment discontinuation in patients with a possible chance for SVR due to a too sensitive assay...
...Moreover, the use of a highly sensitive HCV RNA detection assay at week 24 seems to identify more patients with a future lack of SVR than measurement by RT-PCR...
...Whether patients with low HCV RNA concentrations (i.e. TMA positive/RT-PCR negative) may benefit from prolongation of antiviral therapy should be addressed in future prospective studies..."
ARTICLE TEXT
To the Editor:
For patients with chronic hepatitis C virus (HCV) infection reliable rules for discontinuation of interferon-¿ based antiviral therapy have been proposed in recent consensus statements [1,2]. In HCV genotype 1 infected patients with a HCV RNA decline of less than 2 log steps at treatment week 12 and/or an absolute amount above 30,000IU/ml therapy can be discontinued due to lack of sustained virologic response (SVR) in 98-100% of cases [3-6]. In patients, who achieve an incomplete response at week 12 with a HCV RNA decline of at least 2 log but still detectable HCV RNA, retesting at week 24 is recommended and treatment should only be continued, if HCV RNA is qualitatively negative at week 24 42. However, those recommendations are based on studies in which HCV RNA was measured at week 24 with assays with lower detection limits of 30-50IU/ml (Superquantª, NGI, Los Angeles, CA, USA; Cobas Amplicorª HCV, Roche Diagnostics, Pleasanton, CA, USA).
At present, highly sensitive HCV RNA detection assays (Versantª HCV RNA Qualitative Assay (TMA), Bayer, Emeryville, CA, USA; Cobas TaqManª HCV, Roche Molecular Systems, Pleasanton, CA, USA) are available with lower detection limits of 5-10IU/ml [7,8]. The reliability of these highly sensitive HCV RNA tests for predicting lack of SVR at week 24 of therapy is unknown.
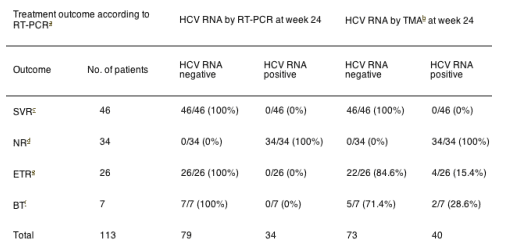
We analysed HCV RNA from -80¡C stored serum samples with a highly sensitive HCV RNA assay (Versantª HCV RNA Qualitative Assay (TMA), Bayer; lower detection limit, 5-10IU/ml) at week 24 of antiviral therapy with (pegylated) interferon-¿ and ribavirin in 113 patients with chronic hepatitis C genotype 1 infection. Original qualitative HCV RNA measurements for assessment of virologic response were performed by RT-PCR (Cobas Amplicorª HCV, Roche Diagnostics, Mannheim, Germany; lower detection limit, 50IU/ml). At treatment week 24 all patients with SVR (46/46) tested negative for HCV RNA by RT-PCR and TMA. 67 patients did not achieve SVR (Table 1). At week 24 HCV RNA was detected by RT-PCR in 34 of those 67 patients (51%). All patients with positive HCV RNA by RT-PCR and an additional 6 patients were positive for HCV RNA at week 24 by TMA (40/67; 60%). None of those 6 patients showed SVR after a 48 week antiviral combination therapy. In 2 patients virologic breakthrough was observed during therapy and 4 patients exhibited a virologic relapse after the end of therapy (Table 1). Thus, compared to RT-PCR testing, 6 additional patients of the 67 patients without SVR (9%) could be identified by the use of TMA at week 24.
Table 1. Highly sensitive HCV RNA measurement (TMA) for discontinuation of therapy at week 24 in hepatitis C genotype 1 infected patients
|
|
 |
| |
aRT-PCR: Cobas Amplicorª HCV, Roche Molecular Systems, Mannheim, Germany; lower detection limit: 50IU/ml.
bTMA, transcription-mediated amplification; VERSANT HCV RNA Qualitative Assay, Bayer, Emeryville, CA, USA; lower detection limit: 5-10IU/ml.
cSVR, sustained virologic response, negative HCV RNA by RT-PCR at the end-of-treatment and 24 weeks thereafter.
dNR, nonresponse, positive HCV RNA by RT-PCR at treatment week 24.
REFERENCES
12. National Institutes of Health Consensus Development Conference Statement: Management of hepatitis C: 2002ÑJune 10-12, 2002. Hepatology 2002;36:S3-S20.
22. Consensus conference. Treatment of hepatitis C. Agence Nationale d'Accreditation et d'Evaluation en Sante (ANAES). Gastroenterol Clin Biol. 2002;26:B303-B320.
32. Berg T, Sarrazin C, Herrmann E, Hinrichsen H, Gerlach T, Zachoval R, et al.. Prediction of treatment outcome in patients with chronic hepatitis C: significance of baseline parameters and viral dynamics during therapy. Hepatology. 2003;37:600-609. MEDLINE | CrossRef
42. Davis GL, Wong JB, McHutchison JG, Manns MP, Harvey J, Albrecht J. Early virologic response to treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C. Hepatology. 2003;38:645-652. MEDLINE | CrossRef
52. Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, et al.. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. CrossRef
62. Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al.. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965. MEDLINE
72. Ross RS, Viazov SO, Hoffmann S, Roggendorf M. Performance characteristics of a transcription-mediated nucleic acid amplification assay for qualitative detection of hepatitis C virus RNA. J Clin Lab Anal. 2001;15:308-313. MEDLINE | CrossRef
82. Sarrazin C, Gartner B, Welker M, Traver S, Zeuzem S. Evaluation of a new, highly sensitive, real time PCR based assay for quantification of HCV RNA. J Hepatol. 2004;40:150A.
|
|
| |
| |
|
|
|