| |
Treatment with peg-interferon alfa-2b and ribavirin of hepatitis C virus-associated mixed cryoglobulinemia: a pilot study
|
| |
| |
Journal of Hepatology
May 2005
Cesare MazzaroadCorresponding Author Informationemail address, Francesca Zoratb, Manuela Caizzib, Carlo Donadac, Giampiero Di Gennarod, Luigino Dal Masoe, Giorgio Carnielloa, Luigi Virgolinia, Umberto Tirellid, Gabriele Pozzatob
a Department of Internal Medicine, Pordenone General Hospital, Via Montereale 24, 33170 Pordenone (PN), Italy
b Department of Internal Medicine and Neurology, University of Trieste, Cattinara General Hospital, Trieste (TS), Italy
c Department of Internal Medicine, Gorizia General Hospital (GO),Italy
d Division of Oncology, Centro di Riferimento Oncologico, Aviano (PN), Italy
e Epidemiology and Biostatistic Unit, Centro di Riferimento Oncologico, Aviano (PN), Italy
ABSTRACT
Background/Aims
The aim of this study is to verify the efficacy and safety of peg-interferon alfa-2b in combination with ribavirin for initial treatment of HCV-associated mixed cryoglobulinemia.
Methods
Eighteen patients (7 women and 11 men) affected by mixed cryoglobulinemia were included in the study and treated with peg-interferon alfa-2b 1.0Êg/kg once a week plus ribavirin (1000mg daily) for 48 weeks, regardless of the HCV genotype.
Results
At the end of the treatment HCV-RNA became undetectable in 15 patients (83%) and most patients improved clinically. One subject suspended treatment at 13th week due to depression. A large fraction of the patients (8 cases: 44%) relapsed both virologically and clinically a few weeks after the end of therapy. At the end of follow-up, only eight patients (44%) obtained a sustained virological response.
Conclusions
Peg-interferon alfa-2b in combination with ribavirin seems safe and useful for patients affected by mixed cryoglobulinemia, but not as effective as in patients with HCV-positive chronic hepatitis without cryoglobulinemia.
Article Outline
o Abstract
o 1. Introduction
o 2. Patients
o 3. Methods
o 3.1. Purpura scoring system
o 3.2. Histology
o 3.3. Virological studies
o 3.4. Therapy
o 3.5. Criteria for therapy evaluation
o 3.6. Follow-up
o 3.7. Statistical analysis
o 4. Results
o 4.1. Biochemical and histological findings
o 4.2. Virological findings
o 4.3. Therapy outcome (Tables 2 and 3)
o 4.3.1. Virological response
o 4.3.2. Biochemical response
o 4.3.3. Immune response
o 4.3.4. Clinical response
o 4.4. Side effects of therapy
o 4.5. Compliance
o 5. Discussion
o References
5. Discussion
This study was performed using a lower peg-interferon alfa-2b dosage in comparison to what was described on HCV updated guidelines [23]. In accordance with our previous experiences, treatment duration was 12 months for all viral genotypes. This therapeutic schedule was adopted in order to enhance drug tolerability and to minimize drop-out rate. Cryoglobulinemic patients are generally older than chronic hepatitis C patients without MC (50±11 years in this series as compared with 42±10 in the paper of Fried and co-workers or with 47±8 in the paper of Heathcote and co-workers) [23,24], and they frequently present extra-hepatic HCV-related manifestations (i.e. glomerulonephritis, polyneuropathy, Sjgren's syndrome) that could be exacerbated by peg-interferons administration [25,26], even with fatal consequences [27]. On the contrary, no cases of extra-hepatic complications are quoted at the base-line in the 1392 patients enrolled in the above-mentioned papers, even in the patients evaluated, but discarded, from those studies. The lower ribavirin dosage, in comparison to current HCV guidelines, was also justified by the frequent appearance of auto-immune haemolytic anaemia (associated with B cell lymphoid proliferation) [28] which could worse the ribavirin-induced hemolysis [29]. However, in consideration of the SVR obtained in this study, this lower dosage regimen could be recommended in patients carrying genotype 2 or 3, who showed a SVR quite similar to that obtained in chronic hepatitis (5/7, i.e. 71%), but the response rate of patients with genotype 1 is rather unsatisfactory (3/11, i.e. 27%) [23]. Taking in consideration the good compliance, the low rate of severe side effects, and, finally, the improvement of the self-assessment of the quality of life, the standard dose of peg-interferon should be recommended in the future therapeutical trials.
This study suggests that peg-interferon plus ribavirin could be considered as the first-line treatment for cryoglobulinemic syndrome: sustained virological response was observed on 44% of patients; the same results were obtained for clinical (purpura and arthralgias disappearance) and biochemical (aminotransferase normalization) responses. Complete immunological response at the end of treatment was observed on all eight patients (44%) that cleared the virus, but cryocrit resumed in two subjects, respectively, at 4th and 8th week during the follow-up. At the end of the follow-up six patients (33%) obtained a complete immunological response. Rheumatoid factor was still detectable in all subjects after treatment; three subjects had a partial response and one relapsed after 8 weeks of treatment.
It is known that HCV chronic stimulation of lymphocyte B clones results in cryoglobulins and rheumatoid factor production [30], but the persistence of cryoglobulins and rheumatoid factor after viral eradication supports the hypothesis that anti-viral treatment interferes partially with lymphocytes B clone activity. In fact, the persistence of subtle immunological alterations (abnormal sIL2r or sCD30 serum levels) has been observed by some authors after the remission of the vasculitis and the HCV-RNA clearance [31]. Alternatively, ÔcrypticÕ HCV replication might be present in the liver or in the immune system (spleen, bone marrow, lymph nodes) giving a viremia below the sensitivity of the available assay, but sufficient to induce a production of RF or cryoglobulins. Taking into account that interferons, besides its anti-viral activity, are endowed with anti-proliferative properties, it is likely that a longer treatment period, perhaps 18 or 24 months, could be necessary to get a complete immunological outcome.
Subject no. 14, affected by membrane-proliferative glomerulonephritis, showed only a transitory response to therapy, with a good reduction of the proteinuria (from 2.1g/24h to 0.9g/24h), but relapsed to 1.9g/day immediately after suspension of the drugs. This observation is in accordance with our previous findings indicating that patients affected by cryoglobulin-related nephropathy are more difficult to treat. Though some recoveries are sporadically reported [32,33], these subjects usually have a very low rate of virus clearance and rarely obtain a remission of membrane-proliferative glomerulonephritis [34-37]. In these cases, new therapeutic strategies are necessary in order to eradicate HCV and to control the renal disease, in fact encouraging results were obtained using the anti-CD20 compound [38,39]. This treatment is safe and well tolerated and adverse events are comparable to standard interferon plus ribavirin combined therapy. This innovative treatment is able not only to improve clinical symptoms, but even to induce remission of the HCV-related low-grade non-Hodgkin's lymphoma [37,39]. The anti-CD20 treatment determines a strong decrease of the immunoglobulin levels, but, despite the induced immunosuppression, a subsequent antiviral treatment seems successful [40].
Neutropenia was observed in two patients (11%); peg-interferon dosage was reduced accordingly, and no therapy interruptions were needed. Anemia is a frequently reported adverse event, as also evidenced in previous ribavirin mono-therapy trials. Haemoglobin decrease, generally of relevant importance, is subsequent to erythrocyte's membrane damage, and could be mainly observed during the first month of treatment. A careful monitoring of patients is therefore necessary. However, no patient needed ribavirin discontinuation due to anemia, while ribavirin dosage was temporarily reduced for three subjects. Only one patient was discontinued for depression, a severe expected adverse event.
Although interferon treatment was sometimes associated with a worsening of immunological outcome of cryoglobulin-associated peripheral neuropathy [41], in our study we observed that two patients, affected by mainly symptomatic peripheral neuropathy, had some clinical benefit from combination therapy. We have not observed the appearance or the worsening of peripheral neuropathy in any subject.
The lower proportion of complete responses, in comparison to chronic hepatitis C patients without MC [42], could indicate that mixed cryoglobulinemia subjects have a more advanced disease coupled with negative prognostic factors (immunological alterations could be blamed for HCV enhanced resistance to anti-viral treatment) [43,44]. Concerning a possible lower efficacy of antiviral therapy in the patients with MC, literature data are not satisfactory: at present 10 studies have been published including 295 nave cases of MC treated with IFN as monotherapy (reviewed by Vassilopoulos and Calabrese) [45] and only 4 studies with 49 cases of relapsed-resistant MC treated with IFN plus ribavirin [21,46-48].
Unfortunately the complete virological response shows a quite wide range, from 4 to 27% with a mean of 16.3% for IFN monotherapy and from 18 to 54% (mean 33%) for combination therapy. To increase the difficulties to compare the results of MC with those of chronic hepatitis, there are differences in IFN alpha type, dosage, and duration of the treatments. The evaluation of the efficacy of combination therapy in MC is even more troublesome, since there are not published data on its efficacy in nave MC patients and, in addition, the limited number of relapsed-resistant MC patients and the absence of randomised controlled trials makes the interpretation of the results rather problematic. Despite these limitations, from these studies on combination therapy in MC, it can be argued that non-responders to IFN show a very poor response to any re-treatment.
To compare this limited number of MC patients with the large number of patients with HCV chronic hepatitis is rather difficult, but SVR is roughly similar for IFN monotherapy, while combination therapy seems less effective in relapser MC than in chronic hepatitis (33% vs 49%) [49]. Our preliminary data with peg-interferon also document a lower response rate than that observed in the most large clinical trials with HCV chronic hepatitis, but the lower dosage regimen should be taken in consideration.
In conclusion, peg-interferon plus ribavirin therapy is safe and more effective than standard interferon+ribavirin in hepatitis C mixed cryoglobulinemia patients. Further clinical research is needed to prove if higher response rates can be obtained with different treatment schedules, such as higher drug dosages or longer treatment periods. New drugs, such as 40K peg alfa2a (which is endowed with a more sustained blood concentrations than peg alfa2b, but with a different distribution volume) [50] and new drug combinations, like peg-interferons plus anti-CD20, should be considered for the future, hoping these new approaches will offer a better understanding of this disease and significant advantages for its therapy.
1. Introduction
Hepatitis C virus (HCV) determines not only chronic liver disease, but is also implicated in several lymphoproliferative disorders [1-5], among them the most common is mixed cryoglobulinemia (MC). The treatment of MC includes several drugs like steroids [6], cyclosporins [7], colchicine [8], plasmapheresis [9] and others [10], but given the documented association with HCV virus, the treatment of choice seems to be the antiviral therapy [11-15]. For chronic hepatitis C, until recently the interferon alfa plus ribavirin was the standard of care [16], but the development of pegylated interferons opened new treatment opportunities [17], even for MC. The aim of this pilot study was to verify safety and efficacy of peg-interferon alfa-2b in combination with ribavirin for initial treatment of HCV-associated MC.
2. Patients
Eighteen patients (7 women and 11 men) affected by MC were included in the study. None of the patients had received antiviral therapy in the past (nave). Diagnosis was based on standard criteria, and showed an active disease, defined as recurrent purpura associated with constant arthralgias and fatigue, despite steroid therapy. In addition to purpura, weakness and arthralgias, eight patients (44%) showed Raynaud's phenomenon, one had Ôsicca syndromeÕ, three had peripheral sensitive neuropathy (17%) and one nephropathy. All patients were Caucasian, heterosexuals and had no history of intravenous drug abuse or ethanol abuse. All patients gave their informed consent before entry into the study, which had been previously approved by the ethic committee of the Region Friuli-Venezia Giulia. In this open-label pilot study, the quality of life was also assessed with the EORTC QLQ-C30 instrument [18].
3. Methods
Values for the liver function tests as well as hematological parameters were determined by usual laboratory methods. Rheumatoid factor (RF), C3 and C4 fractions of complement were measured by rate nephelometry. Cryoglobulin determination was performed according to standard methods as previously reported [19]: the cryoprecipitates, diluted in 0.5M NaCl, were fractionated by high-resolution gel electrophoresis to type cryoglobulins. Individual monoclonal bands were identified by immunofixation after electrophoresis using a cellulose acetate strip impregnated with antibodies specific for heavy and light chains. Mixed cryoglobulins were classified as type II on the basis of the presence of monoclonal IgM immunoglobulins with RF activity complexed with polyclonal IgG, and as type III in the presence of polyclonal immunoglobulins.
3.1. Purpura scoring system
To assess the severity of vasculitis, the following clinical scoring system was used. A score of 0 indicated the absence of skin lesions; a score of 1, the presence of less than 10 purpura spots on the lower leg; a score of 2, the presence of more than 10 spots on the lower leg; a score of 3, the extension of the spots to the upper leg and/or the abdomen; and a score of 4, the presence of skin ulcers and/or gangrene.
3.2. Histology
A liver biopsy was obtained in all but one patients. Samples were placed in buffered formalin, stained with haematoxylin and eosin, and, for reticulum, with Gomori stain. In each biopsy the disease activity and fibrosis were assessed according to METAVIR [20].
3.3. Virological studies
Hepatitis B virus (HBV) and human immunodeficiency virus (HIV) markers were detected by enzyme-linked immunosorbent assay (ELISA) using commercially available kits. The presence of anti-HCV antibodies was assayed by the second generation (four-antigen) immuno-enzymatic screening test ORTHO-HCV (Ortho Diagnostic Systems, Raritan, NJ, USA). The presence of HCV-RNA in the serum was assessed by nested PCR amplification of the conserved 5 untranslated region (5UTR) of HCV. The HCV genotypes were determined with the line probe assay (Inno-Lipa HCV; Innogenetics, Zwijnaarde, Belgium).
3.4. Therapy
Since these patients had a different immunological situation from subjects carrying chronic hepatitis C without CM, we decided to treat all subjects, regardless of HCV genotype, as follows:
-peg-interferon alfa-2b (PegIntron, Schering-Plough,) 1.0Êg/kg once a week (QW) for 48 weeks plus
- ribavirin (Rebetol, Schering-Plough) 1000mg/daily for patients with body weight 65-85kg, or 1200mg for those above this weight, for 48 weeks.
We decided to implement the bucket dosing recommendations for peg-interferon alfa-2b schedule [21], and to use a lower weight-based dosage of 1.0Êg/kg in order to reduce adverse events and to improve the compliance.
3.5. Criteria for therapy evaluation
To overcome the difficulties in finding homogeneous criteria to evaluate different aspects of the disease, as indicated in our previous reports [22], the response to treatment was split into four separate categories: (1) virological response, (2) biochemical response, (3) immune response, and (4) clinical response.
(1) Virological response: effect of treatment on HCV-RNA. Sustained virological response (SVR)-complete response: loss of HCV-RNA at the end of follow-up. Relapse: loss of HCV-RNA at the end of treatment but positivity at the end of follow-up (6 months). No response: persistent positivity during therapy and at the end of follow-up.
(2) Biochemical response: effect of therapy on ALT; normal value for the local laboratory was considered 53IU/l. Complete response: normalization of the serum ALT level during treatment followed by normal ALT values lasting for 6 months after discontinuation of therapy. No response: ALT out of normal value during treatment and follow-up. Relapse: normalization of the serum ALT level during treatment followed by return to abnormal values during follow-up. In some patients this parameter was not considered, since the ALT level was normal at the beginning of the treatment.
(3) Immune response: effect of therapy on serum RF concentration and cryocrit level; normal value for RF was considered as 0-30IU/ml. Complete response: normalization of serum RF concentration and disappearance of circulating cryoglobulins. Partial response: reduction (but not normalization) of RF and cryoglobulins ³50%. No response: Reduction <50% of RF and cryocrit levels or stable levels. Relapse: partial or complete normalization of serum RF and cryoglobulins during therapy followed by return to higher values during follow-up.
(4) Clinical response: effect of therapy on the clinical manifestations of the disease (including purpura, arthralgia and weakness). Complete response: disappearance of all clinical signs of the disease. Partial response: improvement of the clinical symptoms (reduction of the purpura score ³50%). No response: reduction of the purpura score <50% or stable disease. Relapse: partial or complete normalization of clinical symptoms during therapy followed by return to higher score after the end of treatment.
3.6. Follow-up
Biochemical and clinical parameters were determined each month during therapy and every 2 months during follow-up, up to 6 months. Auto-antibodies were determined every 3 months and thyroid function tests every 6 months. Determinations of HCV-RNA were performed before starting the therapy, at 12th week, at the end of treatment (EOT) and at the end of follow-up (EFU). All patients were followed for at least 6 months after the end of therapy.
3.7. Statistical analysis
Descriptive statistics were performed, including proportions, means and standard deviations (SD) of relevant variables.
4. RESULTS
4.1. Biochemical and histological findings
The main clinical, laboratory, and histological findings of patients are captured in Table 1. The age of patients is expressed as years at the start of therapy (mean age 50±11 years). In 3 subjects (17%), no monoclonal component was found; accordingly, in these 3 cases the mixed cryoglobulinemia was defined as type III. A liver biopsy was performed in all patients but one. A chronic liver disease of variable severity was found in all subjects (see Table 1).
4.2. Virological findings
HCV-RNA was detected in all subjects (100%) before therapy. HCV genotyping showed the prevalent presence of genotype 1b (9 patients, 50%; Table 1). Co-infections were not found.
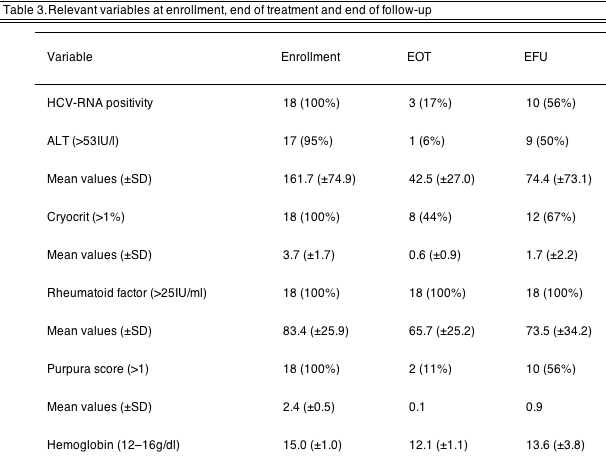
4.3. Therapy outcome (Tables 2 and 3)
4.3.1. Virological response
At the end of treatment (EOT), HCV-RNA became undetectable in 15 patients (83%). At the end of follow-up (EFU), eight patients could be considered as sustained virological responders, 3 non-responders, 6 relapser and 1 drop-out.
4.3.2. Biochemical response
One patient had normal ALT at baseline, hence he was not considered for this assessment. At the EOT 16 patients showed normal ALT levels; at the EFU 8 subjects showed complete biochemical response, 9 were relapser (including 1 drop-out).
4.3.3. Immune response
At the EOT a reduction of cryocrit was observed in 10 patients. Mean cryocrit level was reduced at the EOT, and the reduction, although inferior, was observed also at the EFU. RF showed a completely different pattern: a very low reduction at the EOT, with a rebound at the EFU to almost baseline levels. On the basis of these results, 6 patients can be considered complete responders, 2 partial responders, 7 relapsers and 2 non-responders.
4.3.4. Clinical response
At the EOT 16 subjects were responders, and 2 were partial responders; at the EFU 8 patients were complete responders, 1 non-responder and 9 relapser (including the one withdrawn for side effects). Purpura score dropped at the EOT and partially resumed at the EFU, showing a fashion similar to the cryocrit.
4.4. Side effects of therapy
One patient interrupted treatment after 13 weeks for depression. Hemoglobin mean values decreased during treatment, nevertheless all patients were able to finish treatment with small dose adjustments (see Section 4.5).
4.5. Compliance
A dose reduction of peg-interferon 48-week therapy was required for two subjects; overall dosage of peg-interferon was 81 and 94%, respectively. One patient discontinued therapy after 13 weeks of treatment (27% overall compliance). All the other patients completed therapy with 100% dose compliance.
Ribavirin dose was reduced for 3 subjects, that reached 90, 87 and 92% of the forecasted total dosage for the 48 weeks. One patient discontinued therapy after 13 weeks of treatment (27% overall compliance). All other patients achieved 100% compliance.
The quality of life, assessed with the EORTC QLQ-C30 instrument, showed a significant improvement. When responses at base line were compared with responses at 24th and 48th weeks of treatment, there were significant improvements in the domains of global health status (P<0.001) and physical functioning (P<0.001), while a slight worsening was observed in the domain of emotional functioning (P<0.05), mainly for depression. No significant changes were observed in the domains of cognitive, social and role functioning. Among symptom items, a clear improvement of fatigue (P<0.001) was observed. At the end of the follow-up, the eight patients recovered from infection and from the disease, showed a normal health status, while the non-responders or relapsers returned to pre-treatment levels.
References
1. [1]Agnello V, Chung RT, Kaplan LM. A role for hepatitis C virus infection in type II cryoglobulinemia. N Engl J Med. 1992;327:1490-1495. MEDLINE
2. [2]Ferri C, Greco F, Longobardo G, Palla P, Moretti A, Marzo E, et al.. Association between hepatitis C virus and mixed cryoglobulinemia. Clin Exp Rheumatol. 1991;9:621-624. MEDLINE
3. [3]Pozzato G, Mazzaro C, Crovatto M, Modolo ML, Ceselli S, Mazzi G, et al.. Low-grade malignant lymphoma, hepatitis C virus infection and mixed cryoglobulinemia. Blood. 1994;84:3047-3053. MEDLINE
4. [4]Mazzaro C, Zagonel V, Monfardini S, Tulissi P, Pussini E, Fanni-Canelles M, et al.. Hepatitis C virus and non-Hodgkin's lymphomas. Br J Haematol. 1996;92:1275-1279.
5. [5]Silvestri F, Pipan C, Barillari G, Zaja F, Fanin R, Infanti L, et al.. Prevalence of hepatitis C virus infection in patients with lymphoproliferative disorders. Blood. 1996;87:4296-4301. MEDLINE
6. [6]Vacca A, Dammacco F. Deflazacort versus prednisone in the treatment of EMC: a controlled clinical study. Int Arch Allergy Appl Immunol. 1992;99:306-313.
7. [7]Ballar M, Bobbio F, Poggi S, Bordin G, Bertoncelli MC, Catania E, et al.. A pilot study on the effectiveness of cyclosporine in type II mixed cryoglobulinemia. Clin Exp Rheumatol. 1995;13:201-203. MEDLINE
8. [8]Monti G, Saccardo F, Rinaldi G, Petrozzino MR, Gomitoni A, Invernizzi F. Colchicine in the treatment of mixed cryoglobulinemia. Clin Exp Rheumatol. 1995;13:197-199.
9. [9]Ferri C, Gremignani G, Bombardieri S, Moriconi L, Pontrandolfo A, Vitali C, et al.. Plasma exchange in mixed cryoglobulinemia: the effect on renal, liver and neurologic involvement. La Ricerca in Clinica e in Laboratorio. 1986;16:403-411.
10. [10]Tavoni A, Mosca M, Ferri C, Moriconi L, La Civita L, Lombardini F, et al.. Guidelines for the management of essential mixed cryoglobulinemia. Clin Exp Rheumatol. 1995;13:191-195.
11. [11]Willson RA. The benefit of long-term interferon alfa therapy for symptomatic mixed cryoglobulinemia (cutaneous vasculitis/membranoproliferative glomerulonephritis) associated with chronic hepatitis C. J Clin Gastroenterol. 2001;33:137-140. MEDLINE | CrossRef
12. [12]De Rosa FG, Di Lullo L, Coviello R, Donanno S, Lagan B, Casato M. Interferon-alpha treatment of hepatitis C virus-associated mixed cryoglobulinemia [letter]. J Hepatol. 1998;28:335.
13. [13]Mazzaro C, Lacchin T, Moretti M, Tulissi P, Manazzone O, Colle R, et al.. Effects of two different alpha-interferon regimens on clinical and virological findings in mixed cryoglobulinemia. Clin Exp Rheumatol. 1995;13:180-185.
14. [14]Mazzaro C, Carniello GS, Doretto P, Mazzi G, Crovatto M, Santini GF, et al.. Interferon therapy in HCV-positive mixed cryoglobulinemia: viral and host factors contributing to efficacy of the therapy. It J Gastroenterol Hepatol. 1997;29:343-347.
15. [15]Zuckerman E, Keren D, Slobodin G, Rozenbaum M, Toubi E, Sabo E, et al.. Treatment of refractory, symptomatic, hepatitis C virus related mixed cryoglobulinemia with ribavirin and interferon-alpha. J Rheumatol. 2000;27:2172-2178. MEDLINE
16. [16]McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, et al.. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339:1485-1492. MEDLINE | CrossRef
17. [17]Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al.. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomized trial. Lancet. 2001;358:958-965. Abstract | Full Text | PDF (110 KB) | MEDLINE | CrossRef
18. [18]Aarson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Dueaz NJ, et al.. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality of life instrument for use in international trials in oncology. J Natl Cancer Inst. 1993;85:363-376.
19. [19]Dammacco F, Sansonno D, Piccoli C, Tucci FA, Racanelli V. The cryoglobulins: an overview. Eur J Clin Invest. 2001;31:628-638. MEDLINE | CrossRef
20. [20]Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. Hepatology. 1996;24:289-293. MEDLINE | CrossRef
21. [21]Di Bisceglie AM, Hoofnagle JH. Optimal therapy of Hepatitis C. Hepatology. 2002;36:S121-S127.
22. [22]Mazzaro C, Zorat F, Comar C, Nascimben F, Bianchini D, Baracetti S, et al.. Interferon plus ribavirin in patients affected by hepatitis C virus positive mixed cryoglobulinemia resistant to previous interferon treatment(s). J Rheumatol. 2003;30:1775-1781. MEDLINE
23. [23]Seeff LB, Hoofnagle JH. The National Institute of Health Consensus Development Conference Management of hepatitis C. Clin Liver Dis. 2003;261-287.
24. [24]Heathcote EJ, Shiffman ML, Cooksley WG, Dusheiko GM, Lee SS, Balart L, et al.. Peginterferon alfa-2a in patients with chronic hepatitis C and cirrhosis. N Engl J Med. 2000;343:1673-1680. MEDLINE | CrossRef
25. [25]Gordon AC, Edgar JD, Finch RG. Acute exacerbation of vasculitis during interferon-alpha therapy for hepatitis C-associated cryoglobulinaemia. J Infect. 1998;36:229-230. MEDLINE | CrossRef
26. [26]Boonyapisit K, Katirji B. Severe exacerbation of hepatitis C-associated vasculitic neuropathy following treatment with interferon alpha: a case report and literature review. Muscle Nerve. 2002;6:909-913.
27. [27]Friedman G, Mehta S, Sherker AH. Fatal exacerbation of hepatitis C-related cryoglobulinemia with interferon-alpha therapy. Dig Dis Sci. 1999;44:1. MEDLINE | CrossRef
28. [28]Poggianella M, Benvenuti F, Baracetti S, Nascimben F, Zorat F, Burrone O. HCV-associated autoimmune haemolityc anaemia due to warm-reacting antibodies: evidence of B-cell monoconality [Abstr]. J Hepatol. 2000;32:45. CrossRef
29. [29]De Franceschi L, Fattovich G, Turrini F, Ayi K, Brugnara C, Manzato F, et al.. Hemolytic anemia induced by ribavirin therapy in patients with chronic hepatitis C virus infection: role of membrane oxidative damage. Hepatology. 2000;31:997-1004. MEDLINE | CrossRef
30. [30]Zuckerman E, Zuckerman T, Sahar D, Streichman S, Attias D, Sabo E, et al.. The effect of antiviral therapy on t(14;18) translocation and immunoglobulin gene rearrangement in patients with chronic hepatitis C virus infection. Blood. 2001;97:1555-1559. MEDLINE | CrossRef
31. [31]Lamprecht P, Moosig F, Gause A, Herlyn K, Csernok E, Hansen H, et al.. Immunological and clinical follow-up of hepatitis C virus associated cryoglobulinemic vasculitis. Ann Rheum Dis. 2001;60:385-390. MEDLINE | CrossRef
32. [32]Laganovich M, Jelakovic B, Kuzmanic D, Scukanec-Spoljar M, Roncevic T, Cuzic S, et al.. Complete remission of cryoglobulinemic glomerulonephritis (HCV-positive) after high-dose interferon therapy. Wien Klin Wochenschr. 2000;112:596-600. MEDLINE
33. [33]Yamabe H, Johnson RJ, Gretch DR, Osawa H, Inuma H, Sasaki T. Membrano proliferative glomerulonephritis associated with hepatitis C virus infection responsive to interferon alpha. Am J Kidney Dis. 1995;25:67-69. MEDLINE
34. [34]Mazzaro C, Panarello M, Carniello S, Faelli A, Mazzi G, Crovatto M, et al.. Interferon versus steroids in patients affected by HCV-associated cryoglobulinemic glomerulonephritis. Dig Liver Dis. 2000;32:708-715.
35. [35]Agnello V. Therapy for cryoglobulinemia secondary to hepatitis C virus: the need for tailored protocols and multiclinic studies. J Rheumatol. 2000;27:2065-2067. MEDLINE
36. [36]Bruchfeld A, Lindhal K, Stahle L, Soderberg M, Schvarez R. Interferon and ribavirin in patients with hepatitis C-associated renal disease and renal insufficiency. Nephrol Dial Transplant. 2003;18:1573-1580. MEDLINE
37. [37]Meyers CM, Seef LB, Stehman-Breen CO, Hoofnagle JH. Hepatitis C and renal disease: an update. Am J Kidney Dis. 2003;42:631-657.
38. [38]Zaja F, De Vita S, Mazzaro C, Sacco S, Damiani D, De Marchi G, et al.. Efficacy and safety of Rituximab in type II mixed cryoglobulinemia. Blood. 2003;101:3827-3834. MEDLINE | CrossRef
39. [39]Sansonno D, De Re V, Lauletta G, Tucci AF, Boiocchi M, Dammacco F. Monoclonal antibody treatment of mixed cryoglobulinemia resistant to interferon ¿ with an anti-CD20. Blood. 2003;101:3818-3826. MEDLINE | CrossRef
40. [40]Lamprecht P, Lerin-Lozano C, Merz H, Dennin RH, Gause A, Voswinkel J, et al.. Rituximab induces remission in refractory HCV associated cryoglobulinaemic vasculitis. Ann Rheum Dis. 2003;62:1230-1233. MEDLINE | CrossRef
41. [41]Lidove O, Cacoub P, Hausfater P, Wechsler B, Frances C, Leger JM, et al.. Cryoglobulinemia and hepatitis C: worsening of peripheral neuropathy after interferon alpha treatment. Gastroenterol Clin Biol. 1999;23:403-406. MEDLINE
42. [42]Hadziyannis SJ, Sette H, Morgan TR, Balan V, Diago M, Marcellin P, et al.. Peginterferon-2a and ribavirin combination therapy in chronic hepatitis C. A randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346-355.
43. [43]Rosa D, Saletti G, Valiante N, Barnaba V, Houghton M, Pozzato G. Abrignani S. HCV activates naive B cells via CD81 engagement: a pathogenethic mechanism for B cell disturbances in HCV infection. Proceedings of the 9th international symposium on Hepatitis C virus and related viruses, San Diego, USA; 2002.
44. [44]Lamprecht P, Moosig F, Gause A, Herlyn K, Csernok E, Hansen H, et al.. Immunological and clinical follow up of hepatitis C virus associated cryoglobulinaemic vasculitis. Ann Rheum Dis. 2001;60:385-390.
45. [45]Vassilopoulos D, Calabrese LH. Hepatitis C virus infection and vasculitis: implications of antiviral and immunosuppressive therapies. Arth Rheum. 2002;46:585-597.
46. [46]Calleja JK, Albillos A, Moreno-Otero R, Rossi I, Cacho G, Domper F, et al.. Sustained response to interferon-alpha or to interferon-alpha plus ribavirin in hepatitis C virus-associated symptomatic mixed cryoglobulinemia. Aliment Pharmacol Ther. 1999;13:1179-1186. MEDLINE | CrossRef
47. [47]Donada C, Crucitti A, Donadon V, Chemello L, Alberti A. Interferon and Ribavirin combination therapy in patients with chronic hepatitis C and mixed crioglobulinemia. Blood. 1998;92:2983-2984. MEDLINE
48. [48]Naarendorp M, Kallemuchikkal U, Nuovo GJ, Gorevic PD. Long term efficacy of interferon-alpha for extrahepatic disease associated with hepatitis C virus infection. J Rheumatol. 2001;28:2466-2473. MEDLINE
49. [49]Davis GL, Esteban-Mur E, Rustgi V, Hoefs J, Gordon SC, Trepo C, et al.. Interferon Alfa-2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C. N Engl J Med. 1998;339:1493-1499. MEDLINE | CrossRef
50. [50]Perry CM, Jarvis B. Peginterferon-alpha-2a (40kD): a review of its use in the management of chronic hepatitis C. Drugs. 2001;61:2263-2288. MEDLIN
|
|
| |
| |
|
|
|