| |
Prevention of interferon-alpha associated depression in psychiatric risk patients with chronic hepatitis C
|
| |
| |
Journal of Hepatology
June 2005
Articles in Press
Martin Schaefera, Markus Schwaigera, Andrea S. Garkischa, Maurice Picha, Axel Hinzpetera, Ralf Uebelhacka, Andreas Heinza, Florian van Boemmelb, Thomas Bergb
a Department of Psychiatry, Charité—University Medicine Berlin, Campus Charité Mitte, Schumannstr. 20/21, D-10117 Berlin, Germany
b Department of Gastroenterology and Hepatology, Charité—University Medicine Berlin, Campus Charité Virchow, Augustenburger Platz 1, D-13353 Berlin, Germany
“……The study demonstrates for the first time that pre-emptive treatment with citalopram is highly effective in preventing IFN-α associated major depression in HCV-infected patients irrespective of whether they are patients at risk to develop psychiatric complications or not. Thus, the frequency of IFN-α induced major depression could be reduced up to 40% by applying this kind of pre-emptive treatment…..”
ABSTRACT
Background/Aims: Interferon-alpha (IFN-α)-induced depression is a major limitation for the treatment of chronic hepatitis C, especially for patients with psychiatric disorders. We prospectively studied the efficacy of a pre-emptive treatment with the antidepressant citalopram to prevent depression during hepatitis C treatment with pegylated IFN-α-2b plus ribavirin.
Methods: 14 HCV-infected patients with psychiatric disorders received a prophylactic medication with citalopram (20mg/day) before and during therapy with IFN-α. The incidence of major depression was compared with 22 HCV-infected patients with psychiatric disorders (group B; n=11) and without psychiatric risk factors (group C; n=11), who underwent IFN-α treatment without a pre-emptive antidepressant therapy. Depression was diagnosed by DSM-IV criteria.
Results: Pre-treatment of psychiatric patients with citalopram significantly reduced the incidence of major depression during the first 6 months of antiviral treatment as compared to the two control groups (group A 14% vs. 64% and 55% in group B and C; log-rank 6.89; df=2; P=0.032). Patients who developed symptoms of major depression during IFN-therapy could be also improved by antidepressive treatment.
Conclusions: Our open label pilot study, even small, clearly indicates that IFN alpha induced depression in psychiatric risk patients can be ameliorated by both the use of antidepressants as well as by intensive psychiatric care. However, larger, double blind placebo controlled trials in other patient populations are required to confirm these preliminary findings.
1. Introduction
One of the most important neuro-psychiatric side effects of interferon-alpha (IFN-α) during treatment of chronic hepatitis C virus (HCV) infection is the induction of episodes of major depression and suicidal thoughts [1,2]. In such cases, treatment must be interrupted or at least reduced. On the other hand, adherence to IFN-α therapy is essential to achieving a sustained virological response. Thus, improvement of IFN-α therapy's side effects should lead to a higher compliance rate among patients and have the best antiviral efficacy [3]. However, several reports indicate that IFN-α can also worsen pre-existing affective or schizophrenic disorders [4–6]. This led to the conclusion that patients with psychiatric disorders have an increased risk of developing severe neuropsychiatric complications during IFN-α treatment, e.g. severe depression and suicidal thoughts. From that it was recommended that HCV-infected patients suffering from severe and uncontrolled psychiatric disorders should not be treated with IFN alpha therapy.
Recent investigations showed growing evidence, that besides hormonal and cytokine changes, diminished serotonergic neurotransmission might be involved in the pathoetiology of IFN-associated depressive mood changes [2,6]. Reduced tryptophan levels, increased activity of the indoleamine-2,3-dioxygenase (IDO) and lower activities of serum peptidases, have been associated with an increased risk of developing depressive mood changes during IFN-α treatment [7–11]. Therefore, the application of selective serotonin reuptake inhibitor (SSRI's) seems to be a logical therapeutic strategy for acute treatment or prevention of IFN-associated depression [12–20]. Indeed, the present clinical guidelines in the United States for the management of interferon associated depression during HCV-therapy include evaluating patients for current or past depression, treating depressive symptoms prior to antiviral treatment and conducting close monitoring [21]. At present, however, only one prospective controlled trial has been performed investigating the efficacy of an antidepressant pretreatment for the prevention of depression induced by standard IFN-α-2b in patients with malignant melanoma but without psychiatric disorders [22]. In contrast, no prospective controlled data is available, focusing on the efficacy of a prophylactic antidepressant treatment with patients, who receive antiviral treatment with pegylated interferons because of a chronic hepatits C infection. We, therefore, investigated in an open, prospective and controlled trial, whether a pre-emptive treatment with the SSRI citalopram can prevent major depression during treatment with pegylated interferon-alpha-2b in HCV-infected patients with and without psychiatric disorders.
4. Author Discussion
The study demonstrates for the first time that pre-emptive treatment with citalopram is highly effective in preventing IFN-α associated major depression in HCV-infected patients irrespective of whether they are patients at risk to develop psychiatric complications or not. Thus, the frequency of IFN-α induced major depression could be reduced up to 40% by applying this kind of pre-emptive treatment. A similar observation was made by Musselmann and colleagues who demonstrated, that the therapy with the serotonin reuptake inhibitor paroxetine was very effective in reducing the incidence of major depressive episodes in IFN-α treated patients suffering from malignant melanoma [22]. In contrast to current recommendations [21], our observations clearly indicate a general benefit of an antidepressant pre-treatment for all patients treated with IFN-α, irrespective of the presence of psychiatric risk factors. Furthermore, our study also indicates that the frequency of major depression did not significantly depend on whether patients had already suffered from a psychiatric illness or not. From that one is inclined to propose that patients with psychiatric disorders should not be in general excluded from INF-α therapy. In an interdisciplinary setting, we also recently showed that HCV-infected patients with psychiatric disorders or drug addiction can be successfully treated with IFN-α and ribavirin without an increased risk of an exacerbation of their psychiatric disorder [23]. Similarly, Pariante and coworkers reported, that HCV-infected patients with affective or anxiety disorders did not differ in the frequency and severity of symptoms of depression or drop-outs [24,25]. Van Thiel et al. treated HCV-infected patients with severe psychiatric disorders and drug addiction successfully with an IFN-α monotherapy hereby challenging the concept that a history of psychiatric disorders is a contraindication against interferon alpha therapy [26,27]. However, so far, no trial investigated pharmacological strategies to prevent IFN-α associated psychiatric side effects (i.e. depression) in patients with chronic hepatitis C infection.
Although the incidence of depression in our collective was rather high, it still is comparable to the data reported by other authors using psychiatric diagnostic criteria [15,22,23,28]. There is this interesting observation that trials reported by psychiatrists showed an incidence of depression during IFN-alpha treatment up to 30–60% in contrast non-psychiatric-based trials reporting a frequency of only 20–30% [29,30]. One explanation is, that non-psychiatric trials only realized severe depressive episodes compromising continuation of the antiviral therapy.
The factors which cause depressive mood changes during IFN-α therapy are still not well understood. Beside changes in the metabolism of serotonin, alteration of the physiological stress response HPA-axis and to some extent, genetic factors, may all play a major role in causing the development of depressive symptoms during IFN-α treatment [31–34]. In fact, there is now ample evidence that a blunted (hyper-reactive) HPA-axis is a major factor in the etiopathogenesis of major depression [33]. However, our data strongly support the significance of a central serotonergic deficit and changes in neurotransmission for the development of depression during treatment with interferon-alpha [34–36].
It also can be deduced from our study that citalopram is an effective antidepressive drug for the acute treatment of IFN-α induced depressive symptoms. Patients in both of our control groups B and C who developed the typical symptoms of major depression during IFN-α treatment rapidly responded to a daily dose of 20mg citalopram and recovered from the severe depressive symptoms. Surprisingly, the administration of various kinds of antidepressants was, up to the present, only demonstrated in open and uncontrolled trials or case reports [13–15,17,19] but certainly deserves to be a more widely used concomitant strategy. Our experience is that the application of antidepressants improves the well being of these patients and helps them to cope with their anxieties [12,22,37]. We also could observe during this study that the frequent presence of a devoted psychiatrist is of importance especially for patients with pronounced depressive symptoms and this may also have some implications regarding the consequent adherence to the applied therapeutic regimes. On this basis we are inclined to explain the observed lack of drop-outs caused by psychiatric side effects.
In our trial, weekly dose of pegylated interferon-alpha-2b was only reduced in two patients because of hematological side effects, but in no case because of the development of a major depressive episode. While depressive mood changes during treatment with standard interferons normally disappeared 2–3 weeks after discontinuation of antiviral medication, depressive episodes induced by pegylated interferons persists over weeks to months. Certainly the different half-life of these drugs may be a causative factor in this respect and antidepressant treatment will become even more important for patients treated with pegylated interferons.
However, prospective, randomised, controlled and double blinded trials are needed to clarify the question, if a pre-emptive antidepressant treatment will also protect patients without psychiatric risk factors from IFN-α associated depression. Therefore, data concerning the safety of a concomitant antidepressant therapy and possible influences on viral response to IFN-α treatment has to be generated.
3. Results
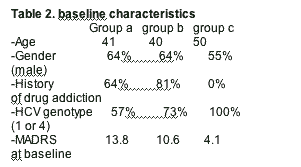
The major clinical parameters of all patients are given in Table 2. The three groups did not differ significantly regarding age or gender, although the control group without a psychiatric disorder tended to be older compared to the psychiatric groups A and B. Overall, MADRS scores at baseline were significantly higher in the psychiatric groups compared to the non-psychiatric controls with the highest values in group A.
3.1. Prevention of IFN-alpha induced major depression
During the 6 month period of IFN-α treatment, major depression was diagnosed by clinical assessment according to DSM-IV criteria in 15 out of 36 patients (42%). While the incidence of major depression in group B (64%) and the control patients (group C) (55%) did not differ significantly, psychiatric patients with citalopram pre-treatment (group A) developed significantly fewer depressive episodes (14%) during the treatment period (95% confidence interval, 0.935–1.731; log-rank test, df=2, P=0.032. Group differences became significant after 4 months of treatment (χ2=6.417, df=2; P=0.040; Kruskall–Wallis test) and remained also significant after 6 months (χ2=7.052, df=2; P=0.029; Kruskall–Wallis test).
Overall, 2 patients in group A, 7 patients in group B and 6 patients in group C developed a major depressive episode during interferon-treatment. However, none of the psychiatric patients had to discontinue interferon-treatment or had to be admitted to a psychiatric hospital due to suicidal thoughts.
3.2. Treatment of IFN-alpha induced major depression
Those patients without antidepressant pre-treatment who developed depression during therapy with IFN-α (n=13; 7 in group B and 6 in group C) received citalopram 20mg/day while the two affected patients in group A were treated with mirtazapine at doses between 30 and 60mg/day in addition to the concomitant therapy with citalopram. Quantitative changes of depressive symptoms were assessed with the MADRS before and after 3 weeks of antidepressant treatment. Mean MADRS scores during major depression were significantly higher in group A (35.0±2.8) and B (26.42±5.1) compared to group C patients (22.5±4.1; F=5.701, df=2, P=0.018; univariate ANOVA with Bonferroni post hoc-test). During the first 3 weeks of antidepressant therapy, the mean MADRS scores decreased significantly from 26.0±5.9 to 12.1±5.1 (T=9.304, df=15, P<0.001). The mean improvement was 55% ±20.1 (range 30.4–94.5%). Overall, 86% (n=13/15) of the patients did response (defined as 40% improvement of the MADRS scale after 3 weeks of antidepressant therapy). No differences were seen between group B and C (response in 6/7 patients in group B and 6/6 patients in group C). However, even the ‘non-reponders’ (one patient in group A and B, respectively) still showed a 30% decrease of MADRS scores after 3 weeks of antidepressant treatment.
Interestingly, MADRS values after 3 weeks of antidepressant treatment did not differ significantly between group A (15.5±4.9), group B (10.8±4.3) and group C patients (12.3±6.2) (F=0.621, df=2, P=0.554). Thus, the decrease of MADRS scores was positively correlated with the MADRS scores levels during IFN-alpha induced depression. All patients who developed depressive episodes and who responded to treatment with citalopram or mirtazapine were kept on antidepressants and finished the HCV treatment successfully.
3.3. Response to antiviral treatment
Overall, 50% of the patients had a sustained virological response (SVR), 43% of the patients with genotype 1 or 4 (12/28) and 75% with genotype 2 or 3 (6/8), respectively, 45% (5/11) of the controls (group C), 54% (6/11) of the patients in group B and 43% (6/14) of the pre-treated patients (group A) showed SVR. Differences did not become statistically significant (χ2=1.607, df=2, P=0.448). In two patients from the controls (group C) dose of PEG-IFN-alpha-2b was reduced during the first 24 weeks of treatment because of hematological side effects. The daily dose of ribavirin had to be reduced in 5 patients (2 in group A and 3 in group C, respectively). The PEG-IFN-alpha - or ribavirin doses had not to be reduced between the week 25 and 48 in patients with genotype 1.
3.4. Drop-outs
The trial had to be discontinued in 4 patients: three of them developed somatic side effects, the last patient moved to another town. All patients were infected with HCV genotype 1. None of the patients had to stop treatment because of psychiatric side effects.
2. Methods
Overall, 36 patients with a chronic hepatitis C infection gave their informed consent to participate in this prospective trial and the study was approved by the institutional ethical board of the Charité—University Medicine Berlin.
2.1. Inclusion and exclusion criteria
Inclusion criteria were a detectable serum HCV-RNA level (AMPLICOR®, Roche Diagnostics, Branchburg, NJ) for more than 6 months and elevated alanine aminotransferase (ALT >30U/L, normal <24U/L). General exclusion criteria were the presence of other liver diseases, Child B or C cirrhosis, severe cardiac or neurological diseases, co-infection with hepatitis B or HIV, presence of hepatocellular carcinoma as evaluated by ultrasound and alpha-fetoprotein, autoimmune disorders, a neutrophil count below 1500 per cubic millimetre, and a platelet count below 75,000 per cubic millimetre.
Psychiatric exclusion criteria were any antidepressant therapy in the last 3 months before antiviral treatment was initiated, and the need for any type of psychiatric medication. Thus, patients with the need for prophylactic or long-term medication, including antidepressants, mood-stabilizers or antipsychotics were not included in the trial. Patients with schizophrenia, bipolar disorder, exacerbated personality disorder, dementia or any other organic brain disease were also excluded. Further psychiatric exclusion criteria were ongoing drug abuse (with the exception of a controlled methadone substitution treatment) and a present abuse of alcohol or benzodiazepines. Drug abuse was controlled prior and during the trial by randomized urine testing.
2.2. Patients (treatment groups)
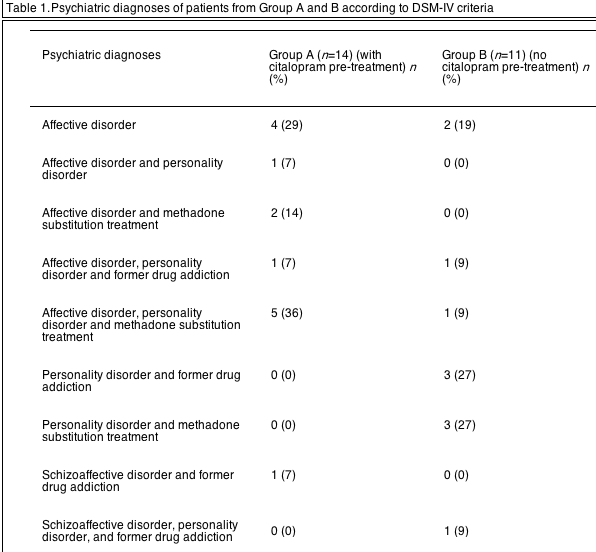
Patients were carefully evaluated by an experienced psychiatrist. Psychiatric disorders were diagnosed using the standardized clinical interview for DSM-IV diagnosis. According to the result of the psychiatric evaluation, patients were distributed into three groups. Group A: patients with the clinical indication of an antidepressant treatment due to current mild to moderate depressive symptoms (n=14). These patients suffered from affective disorders (recurrent depressive episodes) with or without personality disorders and drug addiction. Group B (psychiatric control group): patients with psychiatric disorders but without a clinical need for a present antidepressant treatment (did not present depressive symptoms at baseline or during the last 3 months; n=11). Group C (non-psychiatric control group): patients with chronic hepatitis C in whom a history of psychiatric disorders or drug addiction was carefully excluded (n=11). Psychiatric diagnoses of the patients in group A and B according DSM-IV criteria are listed in Table 1. In most of the patients, the psychiatric diagnose was not verified until they came to our outpatient-clinic. The duration of psychiatric illness (first clinical relevant symptoms or treatment because of drug dependence or psychiatric problems) was estimated according to information received directly from the patients or attending physicians. The mean duration of illness was 4.1±2.2 years in group A and 3.9±1.7 years in group B. Most of the patients reported, that they had no continuous psychiatric treatment in the last 2 years including psychopharmacologic support.
2.3. Treatment regimen
All patients received a combination therapy using pegylated IFN-α-2b (1.5μg/kg per week s.c.) plus ribavirin (800–1200mg/day orally according to body weight). The duration of antiviral therapy was 24 weeks for genotypes 2 (n=2) and 3 (n=6) and 48 weeks for genotypes 1 (n=27) and 4 (n=1). Group A patients received an antidepressive therapy with citalopram (20mg per day per os), starting 2 weeks before the beginning of antiviral treatment. No pre-emptive anti-depressive treatment was applied for patients in group B and C.
2.4. Psychiatric evaluation during treatment
Patients were intensively followed in the psychiatric outpatients clinic over a treatment period of 6 months. All patients were seen by hepatologists and psychiatrists bi-weekly during the first 8 weeks and then once a month. Mental status was continuously monitored. Major depressive episodes during interferon-alpha treatment were diagnosed by clinical assessment according to DSM-IV criteria. In addition, we used the Montgomery Asberg Depression Scale (MADRS) for the quantitative evaluation of depressive mood changes before and during antidepressive treatment. In order to avoid interviewer bias resulting from the lack of a double-blinded setting, the diagnosis of major depressive episodes and MADRS scores were evaluated by one senior psychiatrist who was blinded to the antidepressant pre-treatment as well as to the psychiatric history of the patients. At each visit, patients were additionally screened for suicidal thoughts, irritability, sleeping disturbances, lack of concentration and craving for drugs or alcohol.
2.5. Management of major depressive episodes
In case of major depressive episodes, patients were seen weekly by a senior psychiatrist. Patients without concomitant antidepressant medication (group B and C) who developed depression during antiviral therapy received citalopram 20mg/die with the option of further increase of the daily dosage in case of non-response after 3 weeks of treatment. We started with the same dosage to be able to compare the efficacy of 20mg citalopram regarding prevention and acute treatment of IFN-alpha associated depression. Therapeutic response was monitored with the MADRS before citalopram treatment and after 3 weeks. Patients in group A, who developed a major depressive episode although they had a concomitant treatment with citalopram (20mg/day), received an additional treatment with mirtazapine (30–60mg/day). This different type of antidepressive regimen was chosen in such instances when a patients did not respond to citalopram and also by considering our clinical experience that increase of citalopram dosage is less effective for the treatment of IFN-alpha induced depression than the application of another antidepressive drug as mirtazapine which has as a dual action by enhancing serotonergic as well as noradrenergic neurotransmission. This approach may increase the probability to receive a response even in case of non-response to the SSRI citalopram. Beside the antidepressant treatment, only zopiclon or zolpidem was allowed in case of sleeping disturbances for a period of 14 days or lormetazepam in case of acute anxiety or agitation.
2.6. Statistical analysis
The incidence of depression during IFN-α treatment in the three groups was compared by means of the log-rank test. Survival curves are displayed with Kaplan–Meier plots. Kruskal–Wallis test was used for non-parametric and ANOVA was used to compare parametric data. T-test (two tailed) was used for comparison of the MADRS scores between group A and B after antidepressant treatment.
|
|
| |
| |
|
|
|