| |
Predicting SVR: 12 Better than 4 Week Early Viral Response
|
| |
| |
"Predicting sustained virological responses in chronic hepatitis C patients treated with peginterferon alfa-2a (40KD)/ribavirin"
Journal of Hepatology
June 2005 (Article in Press)
...the results of this analysis provide further support for assessing virological response after 12 weeks of treatment with peginterferon alfa-2a (40KD)/ribavirin, rather than at week 4....although 87% of patients with undetectable HCV viral load at week 4 achieved an SVR, the ability to predict who will NOT achieve an SVR is better after 12 weeks of therapy.... Perhaps the most intriguing new finding of this analysis was the consistent evidence of a relationship between the rapidity of HCV RNA suppression and the likelihood of achieving an SVR... the importance of adherence to therapy was reconfirmed...high relapse rates may indicate SVR might be improved by increasing initial dose of Pegasys and/or prolonging the duration of therapy...
Peter Ferencia, Michael W. Friedb, Mitchell L. Shiffmanc, Coleman I. Smithd, George Marinose, Fernando L. Gonales Jr.f, Dieter Hussingerg, Moises Diagoh, Giampero Carosii, Daniel Dhumeauxj, Antonio Craxik, Monique Chaneacl, K. Rajender Reddym
a Medical University of Vienna, Vienna, Austria
b University of North Carolina, Chapel Hill, North Carolina, USA
c Medical College of Virginia, Richmond, Virginia, USA
d Minnesota Clinical Research, St. Paul, MN, USA
e Prince of Wales Hospital, Randwick, NSW, Australia
f University of Campinas, Campinas, Brazil
g Heinrich-Heine-Universitet, Dusseldorf, Germany
h General Universitario, Valencia, Spain
i Universit degli Studi Brescia, Brescia, Italy
j Hpital Henri Mondor, Creteil, France
k University of Palermo, Palermo, Italy
l Roche, Basel, Switzerland
m University of Pennsylvania, Philadelphia, Pennsylvania, USA
Received 24 November 2004; received in revised form 23 March 2005; accepted 12 April 2005 published online 31 May 2005.
Uncorrected Proof, Article in press
ABSTRACT
Background/Aims
Prediction of sustained virological response (SVR) during treatment would allow clinicians to identify patients most likely to benefit from therapy.
Methods
Retrospective analysis of data from 1121 adults with chronic hepatitis C treated for 48 weeks with peginterferon alfa-2a (40KD) 180mg/week plus placebo or ribavirin (1000/1200mg/day), or interferon alfa-2b 3 MIU three times/week plus ribavirin in a randomized, multinational, study. (Fried study published in NEJM).
Results
Pegasys/RBV (n=453): 58% SVR
IFN/RBV (n=444): 48% SVR
Pegasys+placebo (n=224): 30% SVR
--67% of patients treated with peginterferon alfa-2a (40KD)/ribavirin with early virological responses (HCV RNA negative or ≥2-log10 decrease) at week 12 had SVRs at week 72 (HCV RNA ≦50IU/mL).
--The negative predictive value (NPV) was 97%. The probability of an SVR increased with the rapidity of HCV RNA suppression.
--The highest SVR rates were achieved in patients with rapid virological responses at week 4, but the corresponding NPV (74%) is too low for a decision criterion. Perhaps the most intriguing new finding of this analysis was the consistent evidence of a relationship between the rapidity of HCV RNA suppression and the likelihood of achieving an SVR. Of patients with undetectable HCV RNA after four weeks of treatment with peginterferon alfa-2a (40KD)/ribavirin, 87% achieved an SVR. In those with a ≥2-log10 decrease in HCV RNA levels, but in whom the viral titre remained quantifiable after four weeks, the proportion that developed an SVR was considerably lower (52%). This trend has practical clinical significance and may serve as a tool to individualize treatment.
--In patients with early virological responses by week 12, the SVR rate was 20% lower in those who received <80% compared with patients who received ≥80% of the planned ribavirin dose.
Conclusions
Early, sustained suppression of HCV replication portends an SVR. Cessation of treatment may be contemplated in patients without a ≥2-log10 reduction in HCV RNA after 12 weeks.
Additional Interesting Results:
An SVR was achieved in 9 of 38 patients (24%) who completed peginterferon alfa-2a (40KD)/ribavirin treatment and had a ≥2-log10 reduction in HCV RNA at week 24 but remained HCV RNA-positive (unquantifiable, unknown, or >600IU/mL). Across all treatment groups, an SVR was achieved by 22 of 106 patients (21%) who had this level of HCV RNA reduction by week 24.
Of patients treated with peginterferon alfa-2a (40KD)/ribavirin, 34% (53/158) of those with low baseline HCV RNA levels (≦800,000IU/mL) and 23% (66/289) of those with high baseline HCV RNA levels were HCV RNA negative at week 4. However, the proportion of these patients who achieved an SVR was similar [47/53 (88%) with low baseline HCV RNA levels; 56/66 (85%) with high baseline HCV RNA levels)]. This trend was also apparent in genotype 1 patients, among whom 22% (25/114) of those with low baseline HCV RNA levels and 6% (10/181) of those with high HCV RNA levels were HCV RNA negative at week 4 of peginterferon alfa-2a (40 KD)/ribavirin treatment [SVR was achieved by 22/25 (88%) of those with low baseline HCV RNA levels and 9/10 (90%) of those with high baseline HCV RNA levels).
Baseline HCV RNA level did not affect the predictive value of a ≥2-log10 reduction in HCV RNA at week 4 or 12. In patients treated with peginterferon alfa-2a (40KD)/ribavirin who had a ≥2-log10 reduction but had quantifiable HCV RNA at week 4, 53% (34 of 64 patients) of those with high baseline HCV RNA levels achieved an SVR, compared with 50% (8/16) of those with low baseline HCV RNA levels. Among patients who had had a ≥2-log10 reduction but had quantifiable HCV RNA at week 12, an SVR was achieved in 13% (3/23, 19 of whom were infected with genotype 1) of patients with high HCV RNA levels and no patients (0/6, all genotype 1) with low baseline HCV RNA levels.
Influence of exposure on SVR rates in genotype 1 patients
The influence of the exposure to the study drug on SVR rates could only be investigated in patients with a virological response by week 24 and who had PCR determinations to assess SVR at week 68 or later. This was possible in 70% of genotype 1 patients treated with peginterferon alfa-2a (40KD)/ribavirin. Receipt of ≥80% of the planned ribavirin dose had a significant positive impact on SVR rates in patients treated with peginterferon alfa-2a (40KD)/ribavirin (P=0.0013).
In patients who received ≥80% of the planned peginterferon alfa-2a (40KD) dose, but less than 80% of the planned ribavirin dose, the SVR rate was 20% lower than in those receiving≥80% of the planned ribavirin dose (48% vs 69%, P=0.0143, Table 5). The reduction in SVR rate was similar in the interferon alfa-2b plus ribavirin group (P=0.0560). The percentage of patients with reduced peginterferon alfa-2a (40KD) exposure was low in those with virological responses by week 24. Nevertheless the SVR rate was significantly lower in patients in the peginterferon alfa-2a (40KD)/ribavirin group who had ribavirin dose reductions compared with those who received ≥80% of the planned dose (29% vs 87%, P=0.0064).
In patients infected with HCV genotypes 2 or 3, ribavirin dose reductions after week 24 had no effect on SVR, confirming that 24 weeks of treatment is sufficient in this population (data not shown).
Table 5. Influence of treatment exposure on sustained virological response (SVR) rates in patients infected with hepatitis C virus (HCV) genotype 1 who were treated with peginterferon alfa-2a (40KD) plus ribavirin
All patients 134/208 (64%, 58-71%)
Patients who received ≥80 peginterferon alfa-2a (40KD) and ≥80 ribavirin 100/146 (69%; 60-76%)
Patients who received ≥80 peginterferon alfa-2a (40KD) and <80 ribavirin 19/40 (48%, 32-64%)
Patients who received <80 peginterferon alfa-2a (40KD) and ≥80 ribavirin 13/15 (87%; 60-98%)
Patients who received <80 peginterferon alfa-2a (40KD) and <80 ribavirin 2/7 (29%; 4-71%)
AUTHOR DISCUSSION
Perhaps the most intriguing new finding of this analysis was the consistent evidence of a relationship between the rapidity of HCV RNA suppression and the likelihood of achieving an SVR. Furthermore, the high NPV of the absence of an EVR at week 12 and the importance of adherence to therapy was reconfirmed and serves to reinforce the recommendations in the NIH Consensus Statement [4]. These data can be used to tailor treatment for individual patients.
The high NPV of the absence of an EVR at week 12 remained evident when the data were grouped by HCV genotype or histological status at baseline. The week-12 NPV approached 100% for patients with a high baseline HCV RNA level, although it was somewhat reduced in patients with low HCV RNA level. When all patients treated with peginterferon alfa-2a (40KD)/ribavirin are considered, 86% achieved an EVR, and 58% developed an SVR. A total of 81, 94 and 99% of those infected with HCV genotype 1, 2 or 3, respectively, achieved an EVR with this regimen, and 47, 80 and 80% of patients in each of these subgroups developed an SVR. These findings reflect the well known greater sensitivity of HCV genotypes 2 or 3 to interferon-based therapies. It was recently suggested that genotype 3 patients with high baseline HCV RNA levels may require 48 weeks of therapy [6]. In this study all patients received 48 weeks of treatment, thus we cannot contribute further to this issue. However, data from another large randomized multinational clinical trial suggest that maximal SVR rates can be achieved in this subgroup after 24 weeks treatment with peginterferon alfa-2a (40KD) plus low dose ribavirin [7].
There were also clear differences between treatment groups. The PPV of an EVR was highest in patients receiving peginterferon alfa-2a (40KD)/ribavirin, lowest in those receiving peginterferon alfa-2a (40KD) monotherapy, and intermediate in those treated with interferon alfa-2b plus ribavirin. This finding mirrors the significant differences in SVR rates. Hence the overall outcome of the trial was evident after just 12 weeks of treatment. The optimal time to measure virological responses was also 12 weeks in a study of pegylated interferon alfa-2b plus ribavirin in a similar patient population [5]; however, a lower proportion of individuals achieved an EVR (74%) than in ours (86%). Unlike our study, in which a few patients developed an SVR in the absence of an EVR, no patients in the study by Manns et al [1] achieved an SVR in the absence of an EVR [5].
Beyond the important differences in responses by HCV genotype, the impact of viral factors is difficult to study. Viral eradication requires suppression of replication [8], death of infected hepatocytes and elimination of the virus by an appropriate immune response [9-11]. The extent of viral suppression after four weeks of treatment clearly influences the likelihood of achieving an SVR. Of patients with undetectable HCV RNA after four weeks of treatment with peginterferon alfa-2a (40KD)/ribavirin, 87% achieved an SVR. In those with a ≥2-log10 decrease in HCV RNA levels, but in whom the viral titre remained quantifiable after four weeks, the proportion that developed an SVR was considerably lower (52%). This trend has practical clinical significance and may serve as a tool to individualize treatment.
In each of the three groups, about 10% of patients infected with HCV genotype 1 became HCV negative by week 4 and remained HCV RNA negative for the remainder of the study. These individuals may have HCV strains that are very sensitive to interferon and will respond to any available antiviral treatment regimen. However, at present, no test can identify patients belonging to this subgroup prior to initiation of therapy. The largest difference in the response pattern among the three groups was in patients with a >2-log10 decline in viral load after 4 weeks and HCV negativity at week 12. This group comprised 31% of patients treated with peginterferon alfa-2a (40KD)/ribavirin, but only 20% of patients treated with interferon alfa-2b plus ribavirin and 12% of those treated with peginterferon alfa-2a (40KD)/placebo. This indicates that both the type of interferon-based therapy and the use of ribavirin are important to maximizing SVR rates in this group, and may explain the superiority of peginterferon alfa-2a (40KD) over conventional interferon. The underlying explanation for this phenomenon may involve a third phase of viral clearance, which has been observed after 3 to 6 weeks of combined peginterferon alfa-2a (40KD)/ribavirin therapy [12]. This third phase is more rapid than the second phase of viral elimination and may be essential for viral eradication.
The high relapse rates in patients with a delayed virological response may indicate that treatment was not administered for a sufficient duration. Thus further improvement in SVR rates in patients with genotype 1 might be achieved by increasing the initial dose of peginterferon alfa-2a (40KD) and/or by prolonging the duration of therapy. Higher SVR rates in response to more rapid viral clearance have been obtained with high doses of conventional interferon alfa in prospective randomized controlled trials [13,14]. Prolongation of treatment in slow virological responders (ie in patients with an EVR at week 12 but who remain HCV RNA positive) may decrease relapse rates in end of treatment responders. The feasibility of this approach is being tested in prospective trials. The data also demonstrate that in genotype 1 patients, adherence to therapy after an EVR is important to optimize the probability of achieving an SVR. Our results concur with those of McHutchison et al [15] in that reduced exposure is associated with reduced SVR rates; however, our analysis suggests that early and sustained suppression of HCV RNA is most important in achieving an SVR. Thus, maintenance of exposure to full dose therapy early during the course of treatment is likely to be more important than any arbitrary measure of overall adherence.
On-treatment prediction of non-response allows clinicians to estimate the probability of an SVR during treatment. If a physician obtains a viral load measurement before and after 12 weeks of therapy, treatment may be terminated in non-responders, a policy that reduces the cost of treatment and prevents side effects. Nonetheless, a ≥2-log10 decrease is not an absolute value, and patients with a marked decrease in HCV RNA levels may benefit from continued therapy [16].
In conclusion, the results of this analysis provide further support for assessing virological response after 12 weeks of treatment with peginterferon alfa-2a (40KD)/ribavirin. The importance of full exposure to therapy was also apparent. Most importantly, the data clearly demonstrate the clinical significance of monitoring HCV RNA levels during the course of therapy and the importance of early and sustained suppression of HCV replication.
Introduction
The combination of a pegylated interferon plus ribavirin has produced sustained virological response (SVR) rates up to 63% in patients with chronic hepatitis C [1-3]. The ability to predict, either before or during treatment, whether a patient will achieve an SVR would be of great utility because this combination is more expensive and has side effects similar to conventional interferon/ribavirin. Useful predictors of non-response to therapy must have high negative predictive value (NPV); conversely, useful predictors of an SVR must have a high positive predictive value (PPV).
The observation [2] that patients without an early virological response (EVR) after 12 weeks of treatment with peginterferon alfa-2a (40KD)/ribavirin were highly unlikely to develop an SVR (NPV=97%) was adopted as an assessment criterion by the National Institutes of Health (NIH) Consensus Development Conference [4]. The predictive potential of an EVR has also been confirmed for patients treated with pegylated interferon alfa-2b (12KD) plus ribavirin [5].
The current prediction algorithm is not ideal. The PPV at week 12 was only 65% with peginterferon alfa-2a (40KD)/ribavirin [2]. We hope to refine the treatment algorithm by analyzing virological responses at time points other than week 12, by considering overall exposure to treatment, and by including follow-up data for non-responders. Our results have led to the development of a dynamic prediction model based on the virological response at multiple time points.
RESULTS
Static prediction models
Static prediction models are based on measurement of serum HCV RNA levels at baseline and at a fixed timepoint during treatment.
Predictability by week 12
The NPV of an EVR was consistent across the three groups (97-98%). Among genotype 1 patients treated with peginterferon alfa-2a (40KD)/ribavirin, the PPV of an EVR was 58%, which is significantly higher (P=0.0003) than in those treated with peginterferon alfa-2a (40KD) monotherapy (35%) and slightly higher, but not statistically different from that in patients treated with interferon alfa-2b/ribavirin (55%, P=0.48). When the data are grouped by HCV genotype, baseline HCV RNA level, or histological status at baseline, the trends generally reflect those in the overall population.
In patients treated with peginterferon alfa-2a (40KD)/ribavirin and with high baseline HCV RNA levels, the week-12 NPV was 100% regardless of HCV genotype. Across all treatment groups, the week-12 NPV was lower (89-95%) in patients with low baseline HCV RNA levels than in those with high baseline HCV RNA level.
Of patients who completed therapy, 5.4% (19 of 353), 12.2% (37/304) and 11.2% (17/152), of those treated with peginterferon alfa-2a (40KD)/ribavirin, interferon alfa-2b plus ribavirin, and peginterferon alfa-2a (40KD) monotherapy, respectively, did not have an EVR at week 12. Nevertheless, six of these 73 patients (8.2%, two per group) developed SVRs. Only one of these six individuals had a baseline viral load >800,000IU/mL (range 35,890 to 902,800IU/mL); four patients harbored genotype 1 and one each genotypes 2 and 4. All six patients had a >50% reduction in HCV RNA at week 12, and the four genotype 1 patients had undetectable HCV RNA at week 24.
Comparative predictability at weeks 4, 12 and 24
The likelihood of achieving an SVR was highest in patients who had an RVR at week 4. In patients treated with peginterferon alfa-2a (40KD)/ribavirin who did not have a ≥1-log10 decrease in HCV RNA by week 4 (n=79), the NPV was 78%. The NPV increased between week 4 and 12 but not between weeks 12 and 24, at which times the NPV ranged from 97 to 100% across the three groups. In contrast, the PPV of a virological response did not decrease substantially between weeks 4 and 24, and ranged from 66 to 75% in patients treated with peginterferon alfa-2a (40KD)/ribavirin.
Dynamic prediction models
Dynamic prediction models are based on sequential sampling of HCV RNA before and during treatment and provide information about the rapidity of viral clearance. Among patients treated with peginterferon alfa-2a (40KD)/ribavirin, 87% (104/120) of those who were HCV RNA negative by week 4 developed an SVR. In contrast, an SVR occurred in 52% (43/82) of those whose HCV RNA level dropped by ≥2-log10 by week 4, but remained quantifiable (600IU/mL).
In patients treated with peginterferon alfa-2a (40KD)/ribavirin, SVR was achieved in 91% of those who were HCV RNA-negative at weeks 4, 12, and 24 compared with 60% of patients who had a relatively small reduction in HCV RNA levels (<2-log10) at week 4 but were HCV RNA-negative at weeks 12 and 24; SVR rates were lower for patients who did not achieve HCV RNA negativity until week 24, and lower still for those who had detectable HCV RNA at week 24.
The percentage of genotype 1 patients who became HCV RNA-negative within 4 weeks and remained negative throughout the subsequent 20 weeks was low (approximately 10%) across the three treatment groups. Most of these patients developed an SVR (peginterferon alfa-2a (40KD)/ribavirin 91%, interferon alfa-2b plus ribavirin 91%, peginterferon alfa-2a (40KD)/placebo 93%). However, between weeks 4 and 12 HCV RNA negativity was achieved in 38, 23, and 18% of patients treated with peginterferon alfa-2a (40KD)/ribavirin, interferon alfa-2b plus ribavirin, and peginterferon alfa-2a (40KD)/placebo, respectively, of whom 70, 82 and 44% developed SVRs. Consequently, the percentage of 24-week non-responders was lower in the peginterferon alfa-2a (40KD)/ribavirin group (21%) than in the two other groups (38% of interferon alfa-2b/ribavirin recipients, and 35% of peginterferon alfa-2a (40KD) monotherapy recipients).
PATIENTS & METHODS
This analysis is based on data from a randomized, phase III study of 48 weeks of treatment in patients with chronic hepatitis C with peginterferon alfa-2a (40KD) (PEGASYS®, Roche, Basel, Switzerland) 180mg/week plus ribavirin (COPEGUS®, Roche) 1000 or 1200mg/day (for bodyweight <75 or ≥75kg, respectively), peginterferon alfa-2a (40KD) plus placebo, or interferon alfa-2b 3 MIU three times/week plus ribavirin given as described above. The study design and primary results have been published [2]. The protocol of the original study was approved by the ethics committees at each study site and all patients provided informed consent.
HCV RNA levels were assessed by a qualitative polymerase chain reaction (PCR) assay (COBAS AMPLICOR HCV TEST, v2.0, Roche Diagnostics; detection limit<50IU/mL [100 copies/mL]), and quantified by the COBAS AMPLICOR HCV MONITOR Test, v2.0, (Roche Diagnostics; detection limit<600IU/mL) at baseline and week 4, 12, 24, and 48 of treatment, and at week 12 and 24 of follow-up. Patients with positive qualitative tests during treatment or follow-up were retested with the less sensitive quantitative assay. Treatment was to be stopped in patients with detectable HCV RNA at week 24, in those who missed ≥4 consecutive doses, or at the investigators' discretion [2].
Definitions
Virological response
The various definitions of virological responses are presented in Table 1.
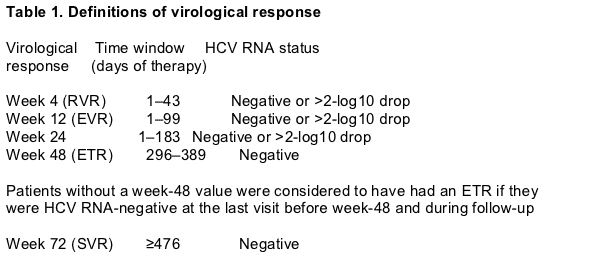
Post- follow-up PCR data were sought for all patients lost to follow-up during the trial. If such patients were confirmed to be PCR-negative they were counted as sustained virological responders in this analysis
|
|
| |
|
|
|