| |
Noninvasive markers of hepatic fibrosis: Are they ready for prime time in the management of HIV/HCV co-infected patients?
|
| |
| |
Download PDF Version
Journal of Hepatology
July 2005
The main study article follows this Editorial below.
Marija Zeremski, Andrew H. Talal
Weill Medical College of Cornell University, New York, NY USA
Study authors: "....We would suggest that the non-invasive fibrosis markers could be performed at the initial evaluation and that patients whose score was below 0.3 could be offered treatment at the discretion of the physician and patient or followed clinically..... In conclusion, in this HIV/HCV co-infected clinic based cohort, 50% of patients with mild hepatic fibrosis could be identified by the SHASTA index. If these results are confirmed, serum fibrosis markers may be used to identify persons with a low stage of liver disease who could be followed conservatively allowing resources to be focused on those with greatest need...... in this study, all 35 subjects with favorable results for the SHASTA index had Ishak fibrosis scores <3, and these represented half of those in that low fibrosis group and one third of those in the study. If confirmed, these results suggest that liver biopsies could have been avoided in one third of all subjects by use of these serological tests. The relative simplicity of adding a quantitative ELISA for HA to AST and serum albumin is attractive for both a cross-sectional diagnostic test and for disease monitoring....."
Hepatitis C virus (HCV) infection is associated with increased morbidity and mortality in HIV-infected patients; up to one-third of HIV-infected patients are co-infected with HCV. In addition, HIV alters the natural history of HCV as indicated by increased serum HCV RNA concentration, accelerated fibrosis progression, and increased prevalence of end stage liver disease and hepatocellular carcinoma [1]. In chronic HCV infection, only a minority (〜20–30%) of patients will progress to fibrosis and cirrhosis over a period of 20–30 years [2]. In HIV/HCV co-infection, fibrosis progression is increased two-fold [3]; over a period of 10–15 years, 15–25% of co-infected patients will develop cirrhosis compared to 2.6–6.5% of HIV negative patients [4,5].
In most patients with chronic HCV infection, a dynamic process is established whereby inflammatory cells are attracted to the liver in an attempt to clear infected hepatocytes. Retention and prolonged survival of intrahepatic inflammatory cells can promote tissue damage that can eventually lead to liver fibrosis. Fibrosis, an excessive accumulation of collagen and other extra-cellular matrix (ECM) proteins, is part of a wound healing process that develops in response to liver injury. Progressive ECM deposition alters hepatic morphology and function ultimately culminating in hepatic impairment, portal hypertension, and the clinical sequela of end stage liver disease. The rate of fibrosis progression varies considerably between patients. Immunodeficiency, as in patients infected with HIV and post-transplantation, steatosis, and alcohol use are important co-factors that promote accelerated fibrogenesis.
Fibrosis assessment is a necessary step in determining the severity of HCV-associated liver disease and whether treatment is indicated. The majority of studies have modeled longitudinal rates of fibrosis progression using cross-sectional data and have assumed linear rates of fibrosis progression, which has not been demonstrated [6]. However, hepatic fibrosis is most likely a dynamic process that remains stable for long periods of time, but which may be marked by flares during which transient bursts of lobular inflammation may result in fibrosis progression [7]. Patients at high-risk for fibrosis progression, such as HIV/HCV co-infected patients, are particularly likely to benefit from frequent fibrosis assessment in order to identify patients with rapidly progressive disease, patients who are candidates for therapy, and patients in whom fibrosis regresses in response to treatment. If fibrosis assessment could be made noninvasively, it could be used as an indicator of treatment efficacy as a supplement to HCV RNA testing.
The lack of accurate, reproducible and easily applied methods for fibrosis assessment has been a limitation in the clinical management and research in hepatitis C. For the past 50 years, liver biopsy has been considered the gold standard for the assessment of hepatic histology. However, the procedure has several disadvantages including cost, morbidity, and mortality. At the same time, the procedure is not without significant limitations. Since a biopsy represents 1/50,000 of the liver, inadequate sample size and the heterogeneity of liver fibrosis in HCV can lead to significant bias in the assessment of hepatic histology. Comparing the right and left lobes of the liver, or even specimens obtained from the same puncture site, the fibrosis stage can differ by at least one stage in up to one-third [8] or even one-half of patients [9]. Specimens of at least 2.5cm in length are recommended to decrease variability associated with inadequately sized specimens [10]. Inter- and intrapathologist variability in the assessment of fibrosis and the use of different scoring systems for fibrosis assessment are additional potential sources of bias [8,11]. Thus, given the inability to frequently perform and the potential risks of liver biopsy, noninvasive methods to assess hepatic fibrosis are urgently needed.
The ideal marker of fibrosis should have high sensitivity and specificity, be readily available, safe, inexpensive, reproducible and able to follow disease progression. Several noninvasive markers of hepatic fibrosis have been evaluated for their ability to assess liver histology (Table 1). Some of them, such as the aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio, the platelet count and the prothrombin index represent measurements routinely obtained in the clinical evaluation of patients. Other markers correspond to components associated with ECM turnover including ECM precursors and components, ECM degradation products, ECM degradation enzymes, and inhibitors of ECM degradation enzymes. Acute phase proteins such as apolipoprotein A1 and haptoglobin, which are nonspecific markers of inflammatory conditions, have also been used to evaluate liver fibrosis. The majority of the currently available markers that are used to assess hepatic histology in chronic HCV patients are shown in Table 1. Although these markers individually may assess hepatic histology with modest reliability, their combination into multicomponent indices generally improves their diagnostic accuracy. The performance characteristics of commonly used indices for fibrosis assessment, such as the Fibrotest (Biopredictive, Paris, France), AST to platelet ratio index (APRI), and Fibrospect (Prometheus Laboratories Inc., San Diego, CA, USA), are illustrated in Table 2.
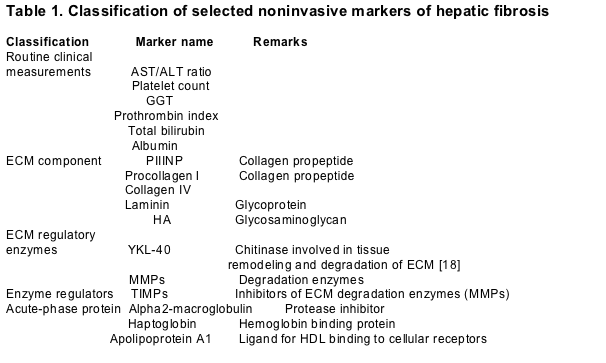
ECM, extra-cellular matrix; AST, aspartate aminotransferase; ALT, alanine aminotransferase; GGT, gamma glutamyltransferase; PIIINP, procollagen III N terminal peptide; HA, hyaluronic acid; MMPs, matrix metalloproteinases; TIMPs, tissue inhibitors of metalloproteinases; HDL, high-density lipoprotein.
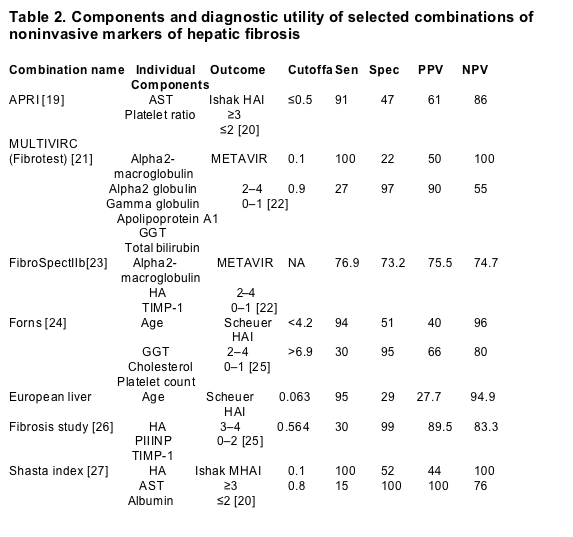
Sen, sensitivity; Spec, specificity; PPV, positive predictive value; NPV, negative predictive value; APRI, AST to platelet ratio index; AST, aspartate aminotransferase; GGT, gamma glutamyltransferase; HA, hyaluronic acid; TIMPs, tissue inhibitors of metalloproteinases; PIIINP, procollagen III N terminal peptide; MHAI, Ishak modified histological activity index scoring system (scale 0–6); NA, not applicable.
aCutoff obtained from receiver operating characteristic (ROC) curve.
bTest performed by Prometheus Laboratories, San Diego, California, US
Ultrasonography is another noninvasive approach used to assess fibrosis. While technically a more challenging method to monitor fibrosis than serum markers, it may have the advantage of assessing the entire liver. Although the sensitivity of ultrasound may be as high as 90% in detecting cirrhosis, the technique is still not used extensively [12,13]. Recently, another ultrasonographic technique based upon liver stiffness, transient elastography (FibroScan; Echosens, Paris, France), has been used to assess liver fibrosis [14]. This technique uses both ultrasound and low-frequency elastic waves whose propagation velocity is directly related to liver elasticity. Transient elastography was shown to have similar accuracy to the Fibrotest and APRI in detecting advanced fibrosis and cirrhosis (METAVIR fibrosis stage ≥2). Combining transient elastography with the Fibrotest resulted in the most accurate detection of fibrosis [15].
In HIV/HCV patients, serum fibrosis markers have received little attention. Myers et al. [16] analyzed the utility of Fibrotest markers (Table 2) in combination with gender, age, and CD4+ cell count to detect advanced liver fibrosis (METAVIR stage ≥2). The authors reported that the combination of alpha2-macroglobulin, apolipoprotein A1, gamma glutamyltransferase (GGT) and gender had the greatest utility in predicting septal fibrosis. In this issue of the Journal, Kelleher et al. [27] assessed liver fibrosis in 95 co-infected patients by analyzing six serum fibrosis markers, including ALT, AST, albumin, total bilirubin, hyaluronic acid (HA), and YKL-40. HA and YKL-40 both are components of ECM. HA is a glycosaminoglycan [17]. YKL-40, a glycoprotein and a member of the chitinase family, contributes to ECM remodeling and degradation [18]. Fibrosis was assessed using the Ishak modified histological activity index (MHAI) scoring system (scale 0–6). HA in combination with AST and albumin (SHASTA Index) was the most accurate for the differentiation between mild (MHAI ≤2) and advanced fibrosis (MHAI ≥3). The index performed very reliably at either end of the disease spectrum. Using a cutoff of 0.1 or 0.8 on the receiver operating characteristic (ROC) curve, the index had 100% accuracy in identifying patients with either mild or advanced fibrosis, respectively. Of the 95 patients in the study, liver biopsies could be avoided in 40 by using a cutoff of 0.1 or 0.8. Among the 40 patients, the vast majority (90%) were in the mild fibrosis category.
Because of their safety and relative ease of application, noninvasive markers of hepatic fibrosis hold certain advantages over liver biopsy in determining fibrosis progression rates in individual patients. For example, in chronic hepatitis C, our understanding of the rate and determinants of fibrosis progression is incomplete because of the difficulty in performing studies utilizing serial liver biopsies. Noninvasive markers can be particularly useful in special populations, such as HIV/HCV co-infected patients, in whom the fibrosis progression rate is increased. Although these markers have had limited assessment in HIV/HCV co-infected patients, the performance characteristics of individual markers, such as HA, and multicomponent indices, such as the Fibrotest, are comparable to those obtained in HCV mono-infected patients. In these patients, additional factors, such as the hepatotoxic effects of antiretroviral medications and hepatic steatosis should also be considered when assessing the diagnostic accuracy of noninvasive markers.
To determine the marker combination with the greatest accuracy, prospective comparative studies utilizing available noninvasive markers with demonstrated utility are needed. Prospective studies could confirm the performance characteristics of noninvasive markers, since the diagnostic accuracy of these markers has largely been assessed using retrospective studies. These studies could also enhance our understanding of the rate of fibrosis progression and assess the improvement in these markers in cirrhotic patients who successfully resolve HCV infection after treatment. Studies in well-characterized HIV/HCV co-infected patients could address whether the inclusion of HIV-specific components, such as HIV RNA or CD4+ cell counts, could improve the diagnostic accuracy of noninvasive fibrosis indices in these patients. The ease with which noninvasive markers can be obtained holds tremendous promise for longitudinal evaluation of fibrosis in patients with chronic liver disease.
Currently, noninvasive markers of hepatic fibrosis can effectively identify patients with either mild or advanced liver disease. In clinical practice, these markers could be the initial step in patient evaluation. In patients with either very low or very high values, liver biopsies could be avoided. Those with intermediate values should undergo liver biopsy. The article by Kelleher et al. in this issue of the Journal moves the field forward by enhancing our understanding of the importance of noninvasive markers in HIV/HCV co-infected patients. The finding that 42% of subjects in their study could potentially avoid liver biopsies indicates that these markers have utility in the evaluation of HCV in HIV-infected patients. However, improvement in the diagnostic accuracy of these markers is required before they can completely supplant liver biopsies.
References
1. [1]Gonzalez SA, Talal AH. Hepatitis C virus in human immunodeficiency virus-infected individuals: an emerging comorbidity with significant implications. Semin Liver Dis. 2003;23:149–166.
2. [2]Afdhal NH. The natural history of hepatitis C. Semin Liver Dis. 2004;24:3–8.
3. [3]Benhamou Y, Bochet M, Di Martino V, Charlotte F, Azria F, Coutellier A, et al.. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999;30:1054–1058.
4. [4]Soto B, Sanchez-Quijano A, Rodrigo L, del Olmo JA, Garcia-Bengoechea M, Hernandez-Quero J, et al.. Human immunodeficiency virus infection modifies the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to cirrhosis. J Hepatol. 1997;26:1–5.
5. [5]Sanchez-Quijano A, Andreu J, Gavilan F, Luque F, Abad MA, Soto B, et al.. Influence of human immunodeficiency virus type 1 infection on the natural course of chronic parenterally acquired hepatitis C. Eur J Clin Microbiol Infect Dis. 1995;14:949–953.
6. [6]Rosenberg WM. Rating fibrosis progression in chronic liver diseases. J Hepatol. 2003;38:357–360.
7. [7]Hubscher SG. Histological grading and staging in chronic hepatitis: clinical applications and problems. J Hepatol. 1998;29:1015–1022.
8. [8]Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, et al.. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614–2618.
9. [9]Siddique I, El-Naga HA, Madda JP, Memon A, Hasan F. Sampling variability on percutaneous liver biopsy in patients with chronic hepatitis C virus infection. Scand J Gastroenterol. 2003;38:427–432.
10. [10]Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–1457.
11. [11]Anon . Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. The French METAVIR Cooperative Study Group. Hepatology. 1994;20:15–20.
12. [12]Gaiani S, Gramantieri L, Venturoli N, Piscaglia F, Siringo S, D'Errico A, et al.. What is the criterion for differentiating chronic hepatitis from compensated cirrhosis? A prospective study comparing ultrasonography and percutaneous liver biopsy. J Hepatol. 1997;27:979–985.
13. [13]Aube C, Winkfield B, Oberti F, Vuillemin E, Rousselet MC, Caron C, et al.. New Doppler ultrasound signs improve the non-invasive diagnosis of cirrhosis or severe liver fibrosis. Eur J Gastroenterol Hepatol. 2004;16:743–751.
14. [14]Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, et al.. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–1713.
15. [15]Castera L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, et al.. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–350.
16. [16]Myers RP, Benhamou Y, Imbert-Bismut F, Thibault V, Bochet M, Charlotte F, et al.. Serum biochemical markers accurately predict liver fibrosis in HIV and hepatitis C virus co-infected patients. Aids. 2003;17:721–725.
17. [17]Patel K, Lajoie A, Heaton S, Pianko S, Behling CA, Bylund D, et al.. Clinical use of hyaluronic acid as a predictor of fibrosis change in hepatitis C. J Gastroenterol Hepatol. 2003;18:253–257.
18. [18]Saitou Y, Shiraki K, Yamanaka Y, Yamaguchi Y, Kawakita T, Yamamoto N, et al.. Noninvasive estimation of liver fibrosis and response to interferon therapy by a serum fibrogenesis marker, YKL-40, in patients with HCV-associated liver disease. World J Gastroenterol. 2005;11:476–481.
19. [19]Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al.. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–526.
20. [20]Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al.. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699.
21. [21]Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357:1069–1075.
22. [22]Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289–293.
23. [23]Patel K, Gordon SC, Jacobson I, Hezode C, Oh E, Smith KM, et al.. Evaluation of a panel of non-invasive serum markers to differentiate mild from moderate-to-advanced liver fibrosis in chronic hepatitis C patients. J Hepatol. 2004;41:935–942.
24. [24]Forns X, Ampurdanes S, Llovet JM, Aponte J, Quinto L, Martinez-Bauer E, et al.. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36:986–992.
25. [25]Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13:372–374.
26. [26]Rosenberg WM, Voelker M, Thiel R, Becka M, Burt A, Schuppan D, et al.. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004;127:1704–1713.
27. [27]Kelleher TB, Mehta SH, Bhaskar R, Sulkowski M, Astemborski J, Thomas DL, et al.. Prediction of hepatic fibrosis in HIV/HCV co-infected patients using serum fibrosis markers: The SHASTA index. J Hepatol. 2005;43:78–84.
Prediction of hepatic fibrosis in HIV/HCV co-infected patients using serum fibrosis markers: The SHASTA index
Journal of Hepatology
July 2005
Thomas B. Kelleherb, Shruti H. Mehtaa, Ramakrishnan Bhaskarb, Mark Sulkowskic, Jacquie Astemborskic, David L. Thomasac, Richard E. MoorecCorresponding Author Informationemail address, Nezam H. Afdhalb
a School of Public Health, Johns Hopkins University, Baltimore, MD, USA
b Liver Center, Beth Israel Deaconess Medical Center, Harvard Medical School, 110 Francis Street, Suite 8E, Boston, MA 02215, USA
c School of Medicine, Johns Hopkins University, Baltimore, MD, USA
ABSTRACT
Background: To examine if serum fibrosis biomarkers could accurately identify the stage of liver disease amongst hepatitis C (HCV) and HIV co-infected patients.
Methods: One hundred and thirty seven HIV/HCV co-infected persons were randomly selected from the Johns Hopkins HIV Clinic cohort. Ninety five had complete testing for fibrosis markers in sera collected at the time of liver biopsy. Biopsies were scored according to Ishak modified histological activity index (F0 no fibrosis to F6 cirrhosis). Fibrosis was evaluated against alanine aminotransferase (ALT), aspartate aminotransferase (AST), AST to platelet ratio (APRI), albumin, total bilirubin, hyaluronic acid (HA) and YKL-40.
Results: Sixty nine (73%) had no or portal fibrosis (F0-2) and were compared with remaining subjects (F3-6). Fibrosis scores ≥F3 were found 27 times more often in persons with HA levels >86ng/ml and 5.5 times more often in persons with HA levels 41–86ng/ml. Less substantial associations were detected with levels of albumin <3.5g/dl (OR 4.85) and AST >60iu (OR 5.91). All 35 subjects who had favorable results of HA, albumin, and AST had minimal fibrosis (F0-2).
Conclusions: Amongst HIV/HCV co-infected patients, serum testing for HA, albumin, and AST (SHASTA Index) was able to accurately stage mild and advanced fibrosis.
INTRODUCTION
Chronic hepatitis C is characterized by slowly progressive hepatic fibrosis. The fibrogenic stimulus transforms the quiescent hepatic stellate cells (HSCs) into myofibroblast-like cells which degrade normal extracellular matrix (ECM) with accumulation of collagen [1]. Collagen accumulation results in architectural distortion with portal fibrosis leading to bridging fibrosis and eventually cirrhosis. The currently accepted gold standard in fibrosis determination is liver biopsy [2,3]. Although widely performed and accepted in diagnosing hepatic fibrosis, liver biopsy has many inherent shortcomings.
Percutaneous liver biopsy is invasive with associated morbidity. Approximately 1–3% of patients require hospital admission following biopsy for an associated complication [4]. Furthermore, even with experienced physicians performing the biopsy and expert pathologists interpreting them, our so called gold standard has up to a 20% error rate in staging disease predominantly related to sampling error [5,6].
Ideally serum markers of fibrosis could either replace the need for liver biopsy or identify those patients with more advanced liver disease who may benefit from a liver biopsy. Features essential of such proposed markers include hepatic specificity, ease of determination and ability to discriminate between degrees of fibrosis. Ultimately serum markers may even have the ability to determine response to various therapies and evaluate disease progression [1].
No single marker fulfills all of the proposed criteria to merit routine clinical use. A combination of markers including those that reflect alterations in hepatic synthetic function and markers of extracellular matrix turnover are emerging as useful diagnostic tests for differentiating early from advanced fibrosis [7,8].
Hepatitis C and HIV co-infection is estimated to affect 200,000 people in the US alone [9,10]. Co-infection is associated with more rapid progression of fibrosis, liver failure and hepatocellular carcinoma [11–15]. Consequently, there is an urgent need for reliable markers of fibrosis progression in this patient group. In this study we report the sensitivity, specificity and predictive value of existing putative fibrosis markers in a cohort of HIV/HCV co-infected persons receiving treatment in an urban HIV clinic.
AUTHOR DISCUSSION
Liver biopsy is the predominant diagnostic test upon which determination of prognosis and indeed the need for antiviral therapy is made in patients with chronic hepatitis C although its performance in accurately staging liver disease has recently come into focus. A recent study on virtual liver biopsy has suggested that liver biopsies need to be 25mm long and non-fragmented to accurately stage disease in 80% of patients [20]. However, that goal is rarely achieved in clinical practice (<25% even in expert liver centers) and always achieving it may increase the risk of complications. In addition, in another study, laparoscopic biopsies taken at the same time from both lobes of the liver revealed substantial differences even when sampled under direct observation and on the same day [21]. Therefore, there is an obvious need for reproducible and reliable non-invasive markers of fibrosis.
The predominant indication for liver biopsy in HCV is staging to determine the need for anti-viral therapy, one important goal of non-invasive markers is to be able to discriminate treatment need. In co-infected patients the clinical situation is more complex as a liver biopsy may also be necessary for evaluation of drug induced hepatotoxicity or opportunistic infections. However, the main indication still remains disease staging and accordingly, in this study, we sought to use serum markers to differentiate persons across the fibrosis gradient traditionally used for therapy (MHAI Stage >3).
In our study, seven biochemical tests, each with biological plausibility and supporting literature were considered, most were correlated to some degree with liver fibrosis in our study. The strongest associations were found with levels of HA. Increased serum HA may be a result of increased hepatic stellate cell production as well as a decrease in removal by hepatic sinusoidal endothelial cells. Our data confirm several other studies of HCV infected persons without HIV that detected an association of HA with hepatic fibrosis [22,23]. HA appears to be especially reliable as a predictor of the absence of advanced fibrosis (cirrhosis). In one study of chronic hepatitis C patients a serum HA less than 60ug/l had a 99% accuracy in predicting the absence of cirrhosis on liver histology (negative predictive value), however, conversely its accuracy in diagnosing cirrhosis was low (30% positive predictive value) [23].
In our own cohort, HA levels were even more discriminating for low amounts of fibrosis when combined with albumin and AST results, a fibrosis index that we suggest be named the SHASTA index (Serum HA, AST, Albumin). All 35 subjects with favorable results for the SHASTA index had Ishak fibrosis scores <3, and these represented half of those in that low fibrosis group and one third of those in the study. If confirmed, these results suggest that liver biopsies could have been avoided in one third of all subjects by use of these serological tests. The relative simplicity of adding a quantitative ELISA for HA to AST and serum albumin is attractive for both a cross-sectional diagnostic test and for disease monitoring. In addition, this is only the second cohort study that has focused on evaluating fibrosis markers in patients co-infected with HIV. Interestingly, the Hopkins HIV cohort in this study is really a community based cohort and is without the biases associated with tertiary referral cohorts which often overestimate the degree of fibrosis.
Currently, there are multiple methodologies proposed to evaluate liver fibrosis in HCV and all appear to perform reasonably well and with a similar diagnostic accuracy. The FibroTest (FibroSure, LabCorp) is one of the most commonly utilized tests and probably the best validated test, even in HIV/HCV patients [24]. The optimal AUC for FibroTest is approximately 0.856 and comparable to the 0.878 with SHASTA index. A recent study from Rosenberg and the European Liver Fibrosis Group used HA, procollagen peptide III and TIMP-1 to stage fibrosis in a variety of liver diseases and had again a comparable AUC of 0.804 overall [25]. All of these indices appear to be non-specific and are more fibrosis indices rather than disease specific indices. The SHASTA index in HIV/HCV has similar accuracy to FibroTest and in this study performed significantly better than the APRI test. Validation of the use of these tests in clinical practice algorithms deserves further study.
Studies of fibrosis markers are important in persons co-infected with HIV and HCV. In the United States and Europe, about one quarter of HIV infected persons also have HCV infection [26]. Co-infected patients have more rapid progression of cirrhosis, liver failure and hepatocellular carcinoma[11–14,27–30]. Furthermore, many of those co-infected with both HCV and HIV due to intravenous drug use often have poor access and compliance with available healthcare. Thus, fibrosis marker research needs to be conducted in this setting not only because the pathogenesis of disease appears to differ (or at least accelerated), but also because biopsies are more difficult to obtain.
In this and other studies, the correlation of fibrosis markers and liver histology is not perfect. Not surprisingly, the best correlation is found at the extreme spectra of fibrosis, i.e. minimal fibrosis and cirrhosis. The inability to always identify patients with moderate disease, Ishak stage 2–4 is not surprising since fibrosis is a complex, multidimensional process. Since liver biopsy is only 80% accurate in staging disease, biomarkers can only be accurate to the same extent. Recently, Poynard and colleagues reported that an inadequate biopsy rather than inaccuracy of biomarkers was more commonly the cause for divergent results between FibroTest and biopsy [31].
This relative inadequacy of biopsy specimens has resulted in some authorities suggesting that biomarkers may even be more accurate than biopsy in staging disease [32].
There are several important limitations to our study. First, the cohort is chiefly composed of African—American males of relatively low body weight and who are chiefly infected with genotype 1 HCV infection acquired by injection drug use. Further validation of this fibrosis algorithm should be undertaken in a more diverse HIV/HCV population and also expanded into the HCV monoinfected patients. However, this population reflects a homogeneous cohort of HIV co-infected males that frequently are resistant to liver biopsy and thus represent an appropriate cohort for evaluation of non-invasive markers. Second, ART affects blood levels of liver enzymes [33] and consequently could affect the degree to which biochemical tests predict liver disease. There is no evidence that ART will effect HA or albumin levels but it could impact the AST level. In this study, all patients were on ART and thus it is highly unlikely to have affected the analysis. Utilization of this algorithm in different HIV populations should control for ART therapy.
In reality biomarkers are probably complementary to biopsy and may in fact be additive in helping to correctly classify the degree of fibrosis. We would suggest that the fibrosis markers could be performed at the initial evaluation and that patients whose score was below 0.3 could be offered treatment at the discretion of the physician and patient or followed clinically. Patients with indeterminant scores could have a biopsy and serial fibrosis markers utilized to follow patients. In fact, the optimal role for markers may be to monitor disease progression or therapy. Research in biomarkers needs to focus on their utilization in longitudinal cohort studies and evaluation of their role as markers of prognosis and disease progression.
In conclusion, in this HIV/HCV co-infected clinic based cohort, 50% of patients with mild hepatic fibrosis could be identified by the SHASTA index. If these results are confirmed, serum fibrosis markers may be used to identify persons with a low stage of liver disease who could be followed conservatively allowing resources to be focused on those with greatest need.
RESULTS
Among the 95 subjects, the median age was 45, 63% were male and 94% were African–American (Table 1). The median CD4 cell count was 340 (interquartile range [IQR], 155–523) and the median HIV RNA level was 235 copies/ml (IOR, 31–19,238). The median HCV RNA level was 3,740,000IU/ml (IQR, 1,290,000–5,870,000). No patients were positive for HBV DNA.
3.1. Fibrosis results
Of the 95 subjects, 35 (37%) had no fibrosis (stage 0) and 34 (36%) had minimal fibrosis (stages 1–2). Twenty six (27%) had bridging fibrosis or cirrhosis (F3 or more) (Fig. 1). The associations of serum markers and APRI with fibrosis are shown in Table 2. Those with ≥F3 fibrosis were significantly more likely to have lower levels of serum albumin (<3.5g/dl) and lower platelet counts (<150,000) and higher ALT (>37IU/l) and AST (>60IU/l) levels (P<0.01). Moreover, compared to persons with little or no fibrosis, those with ≥F3 fibrosis had significantly higher serum levels of YKL and HA (P<0.05).
In multivariate analysis, fibrosis scores ≥F3 were found 27 times more often in persons with HA levels >86ng/ml (95% CI 5.11, 138.7) and 5.5 times more often in persons with HA levels 41–86ng/ml (95% CI 1.12–26.8) (Table 3). Distribution of HA scores across each Ishak fibrosis stage are represented in Fig. 2. Less substantial associations were detected with levels of albumin <3.5g/dl (OR 4.85 95% CI 1.24–19.0) and AST >60IU/l (OR 5.91, 95% CI 1.62–21.5). Adjustments for age, gender, alcohol use, body weight and ART use did not substantially alter these associations (data not shown). When these three independent markers (HA, AST, and albumin) were considered as categorical variates as predictors of fibrosis (MHAI 0–2 vs 3–6), the regression model was as follows:Risk score=-3.84+1.70 (1 if HA 41–85ng/ml, 0 otherwise) +3.28 (1 if HA>85mg/ml, 0 otherwise) +1.58 (albumin <3.5g/dl, 0 otherwise) +1.78 (1 if AST >60IU/l, 0 otherwise). The area under a ROC curve was 0.878 (Fig. 3). The area under the ROC curve for APRI was 0.71 and overall the APRI performed less well as a marker for liver fibrosis in this population.
The markers performed best in the extreme categories. For example, a cutoff of 0.8 was associated with a specificity of 100% and a positive predictive value of 100%. So all individuals with scores >0.8 had ≥F3 fibrosis and there were no false positives at this level. However, only four individuals had scores that were in this range. At the other extreme, a cutoff of <0.30 was associated with a sensitivity of >88% and a negative predictive value of >94%. Moreover, all 35 subjects who had favorable results of HA, albumin, and AST had fibrosis scores of 2 or less. It is important to note that cutoffs in between 0.3 and 0.8 performed poorly in terms of sensitivity and specificity. Thus overall 42% of patients could be correctly classified at either extreme but 58% would not be classifiable with scores between 0.3 and 0.8.
We also examined associations between the markers and inflammation and steatosis. In multivariate analysis, albumin <3.5g/dl (OR, 5.0; 95% CI, 1.41–17.6), YKL 24–69ng/ml (OR, 5.26; 95% CI, 1.06–26.0) and YKL >69ng/ml (OR, 6.41; 95% CI, 1.15–35.6) were associated with greater inflammation (MHAI≥5). The area under the ROC curve for these two markers was 0.73. In terms of steatosis, only albumin <3.5g/dl (OR, 2.83; 95% CI, 0.91–8.80) was marginally associated with steatosis (defined as presence of any fat in the liver).
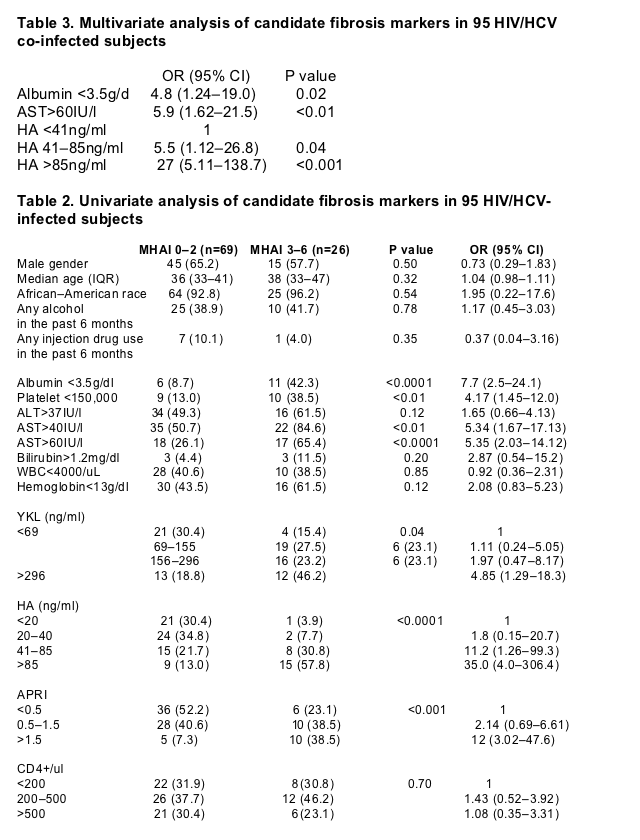

Data is presented as n(%) unless otherwise indicated; P values are from _2 tests for categorical variables and Mann–Whitney tests for continuous variables comparing individuals with MHAI 0–2 vs MHAI 3–6; Odds ratios from univariate logistic regression where outcome is Ishak Fibrosis Score (1 if MHAI 3–6); HIV, human immunodeficiency virus; HCV, hepatitis C virus; OR, odds ratio; CI, confidence interval; IQR, interquartile range; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase; WBC, white blood cell count; HA, hyaluronic acid; APRI, AST to platelet ratio index (calculated as AST[/ULN]*100/Platelet count [109/l].
Materials and methods
The population for this study derives from HIV/HCV co-infected members of the Johns Hopkins University (JHU) HIV clinic cohort in Baltimore, MD. To obtain an unbiased estimate of the prevalence and severity of liver disease among HIV–HCV co-infected persons treated with anti-retroviral therapy (ART), 137 subjects were randomly selected from a group of 630 HIV–HCV co-infected persons who had received ART for two or more years and had not yet received treatment for HCV infection [16]. Of the 137 sampled, 25 individuals were excluded due to the following reasons: undetectable HCV RNA (n=5), medical contraindications (n=5), died before biopsy (n=1), end-stage liver disease (ESLD) (n=3), lost to follow-up (n=5) and other (n=6). After these exclusions, 112 individuals remained. These patients were not substantially different from other eligible individuals who were not selected with respect to age, gender, liver enzymes, CD4 cell count, and HIV RNA level (P>0.05, data not shown). However, patients in the random sample were slightly more likely than the other eligible individuals to be African–American and to have a history of alcohol abuse (P=0.05, data not shown). A total of 95 of these 112 had sufficient stored sera to perform complete fibrosis marker testing.
Demographic and clinical data was obtained prospectively as previously described [17]. Both the Johns Hopkins University Joint Committee on Clinical Investigation and the Beth Israel Deaconess Human Studies Committee approved the study and written informed consent was obtained for all participants.
Laboratory testing
Patients had standard laboratory assessments at each visit performed by licensed clinical laboratories including a complete blood cell count with platelets, serum chemistry panels, alanine aminotransferase (ALT), aspartate aminotransferase (AST), CD4 cell count and plasma HIV RNA level (reverse transcriptase polymerase chain reaction). HCV testing was performed using a second- or third-generation enzyme immunoassay (EIA 2.0, Abbott Laboratories, Abbott Park, IL; EIA 3.0, Ortho Diagnostics, Raritan, NJ) and confirmatory HCV RNA testing (COBAS AMPLICOR MONITOR assay, Roche Diagnostic Systems). Individuals also had quantitative PCR testing for HBV DNA.
Serum fibrosis markers
Markers of liver fibrosis were assessed in serum collected at the time of the liver biopsy. The levels of hyaluronic acid (HA) and YKL-40 were assessed in serum samples, stored at -80°C, using commercially available assays. Hyaluronic acid levels (ng/ml) were determined using the enzyme-linked binding protein assay kits supplied by Corgenix Inc. (Colorado, USA). YKL-40 levels (ng/ml) were determined using METRA YKL-40 EIA kits (Quidel Corporation, San Diego, CA).
Liver histology
Under ultrasound guidance a radiologist performed a transcutaneous liver biopsy with an 18-gauge needle. A single pathologist blinded to all clinical and serological results evaluated all slides. All biopsies were deemed adequate based on specimen size (>10mm) and number of portal tracts (>5) and scored according to the Ishak modified histological activity index (MHAI) scoring system [18]. The mean biopsy size was 11.8mm (SD +2.89mm) with a median of 12mm. The median number of portal tracts was 8 and all biopsies were deemed adequate for pathological interpretation. Steatosis was classified on a five point scale as follows: 0, none; 1, steatosis involving <5% of hepatocytes; 2, 5–<30%; 3, 30–60%; 4>60%.
Statistical analysis
Analyses were designed to differentiate persons with no or ‘minimal’ liver fibrosis (MHAI 0–2) from those with fibrosis, defined as MHAI scores of 3 or more. Univariate associations between markers and fibrosis were examined using _2-tests for categorical variables, Mann–Whitney tests for continuous variables and logistic regression. All markers were examined as continuous and categorical variable. Categories were defined according to the distribution of the marker as well as clinical significance. Commercially available liver enzyme results were examined as a function of the upper limit of ‘normal’ defined by the laboratory, which are based on averages of testing of populations not known to have disease. Research test results (HA and YKL-40) were examined by quartiles in the study population. The AST to platelet ratio index (APRI) was calculated using the formula APRI=AST level(/ULN)*100/platelet count (109/l) and categorized according to cutoffs defined by Wai et al. [19]. Variables that were significant in univariate analysis with a P value <0.15 were considered in multivariate analysis after assessment of multicollinearity by variance inflation factors and tolerance. Variables were entered into a multiple logistic regression model in a backwards stepwise fashion. Those that were significant in the multivariate models with a P value <0.05 were retained. A predictive model was constructed by modeling the independent variables (HA, albumin and AST) as categorical variates and their coefficient of regression. The diagnostic value of the model was assessed by calculating the areas under the receiver operating characteristic (ROC) curves. An area under the curve of 1.0 is ideal whereas a score of 0.5 indicates no diagnostic accuracy.
|
|
| |
| |
|
|
|