| |
Roche Launches New Campaign to Educate Consumers About Hepatitis C
|
| |
| |
Roche issued this press release.
--SEE AD AT END OF THIS ARTICLE--
--Effort Encourages Patients to Speak with Physicians About Available Therapies--
NUTLEY, New Jersey (July 26, 2005) - Roche announced today the launch of a major new campaign to motivate hepatitis C patients who have been diagnosed with the disease to take the critical step of discussing prescription treatment with a liver specialist or hepatitis C-treating physician.
Often called a "silent disease," hepatitis C usually reveals no specific signs or symptoms and remains rarely diagnosed until its chronic stages when it has already caused severe liver disease. In fact, when left untreated, hepatitis C may lead to cirrhosis, liver cancer and even death. According to the Centers for Disease Control and Prevention (CDC), there are approximately four million Americans currently infected with hepatitis C, yet less than 30 percent, or about one million people, have actually been diagnosed with the disease. Additionally, almost 60 percent of those one million diagnosed patients have never been treated.
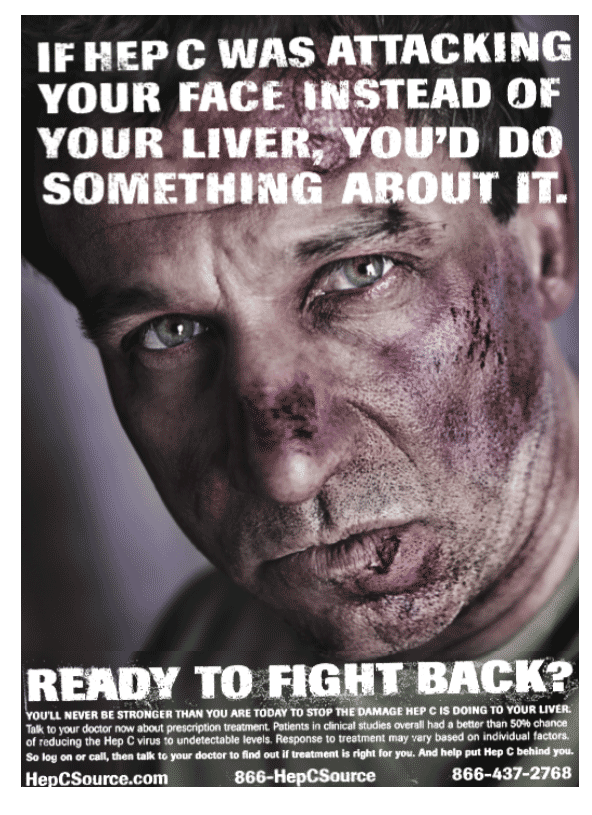
"It is critical for hepatitis C patients to be educated about the potential harmful effects of allowing the disease to go untreated," said Dr. Paul Pockros, Scripps Clinic, San Diego, Calif. "Anyone who has been diagnosed with hepatitis C should speak to a physician to determine whether treatment is medically appropriate." The centerpiece of the campaign is a print advertisement featuring a man with a severely bruised face and a tagline that reads, "If Hep C was attacking your face instead of your liver, you'd do something about it." The ad, which debuts in major national and local daily newspapers today, will begin to appear in national newsmagazines in August and in transit media in September.
There are three versions of the ad, depicting a Caucasian, an African-American, and a Latino.
"As an advocate for those living with hepatitis C, I have seen firsthand the need for increased awareness of the disease. This campaign is an important addition to our own outreach efforts," said Andi Thomas, Executive Director, Hep-C Alert. "Patients should know their options and be proactive in the management of their disease, and this campaign helps achieve those patient education objectives."
The campaign directs consumers to a new Web site,www.hepCfight.com, and to a telephone information line (866-HepCSource), which both serve as patient resources for information about the disease. "As the market leader in the treatment of hepatitis C, Roche is undertaking this new disease awareness campaign to communicate the potential harmful effects of hepatitis C on the liver in a way that consumers can easily recognize and understand," said Gary Ziezula, Vice President, Commercial Operations, Roche.
"Our hope is that the ad will motivate those diagnosed with hepatitis C to make an appointment and speak to their physicians to determine if treatment is the next best step."
More About Chronic Hepatitis C
Hepatitis C is a blood-borne infectious disease of the liver and a leading cause of cirrhosis, liver cancer and the need for liver transplants.
An estimated 2.7 million Americans are chronically infected with the hepatitis C virus (HCV), with 35,000 to 180,000 new infections each year. The CDC estimates that hepatitis C is responsible for eight to ten thousand deaths per year and could increase to 38,000 by the year 2010, surpassing annual HIV/AIDS deaths.
Treatment of Hepatitis C
The combination therapy of pegylated interferon and ribavirin is the current standard of care for hepatitis C. Clinical trials have shown that this combination treatment makes the hepatitis C virus undetectable in more than half of the patients who are treated. Response to treatment may vary based on individual factors, genotype, viral load and race. There is no vaccine available for hepatitis C. Combination therapy results in better treatment responses than monotherapy, but the highest response rates have been achieved with pegylated interferon in combination with ribavirin. Currently, the best indicator of effective treatment is an SVR, defined by the absence of detectable HCV RNA in the serum as shown by a qualitative HCV RNA assay with lower limit of detection of 50 IU/mL or less at 24 weeks after the end of treatment. The following are some of the most common side effects associated with pegylated interferon plus ribavirin therapy: flu-like symptoms, including fever, chills, and muscle aches; fatigue; upset stomach, nausea/vomiting; loss of appetite; difficulty in controlling blood sugar
levels (which may lead to diabetes); skin reactions (such as rash, dry or itchy skin, temporary hair loss, or redness and swelling at the site of injection); temporary hair thinning; and trouble sleeping. Possible serious side effects include mental health problems such as depression, blood problems, infections, and problems with the lungs, eyes, immune system, and heart. Healthcare providers may treat these side effects, change the amount of medication, or stop treatment.
About Roche - More Than a Century in the U.S. and the World
Founded in 1896 and headquartered in Basel, Switzerland, Roche is one of the world's leading innovation-driven healthcare groups. Its core businesses are pharmaceuticals and diagnostics. Roche is one of the world's leaders in diagnostics, the leading supplier of pharmaceuticals for cancer, as well as a leader in virology and transplantation. As a supplier of products and services for the prevention, diagnosis and treatment of disease, the Group contributes on many fronts to improve people's health and quality of life. Roche employs roughly 65,000 people in 150 countries, including approximately 15,000 in the United States.
Roche's U.S. operations celebrate their American Centennial in 2005. In another milestone this year, Roche was named in January toFortunemagazine's list of Best Companies to Work for in America. One of an increasingly rare breed of major healthcare companies that still bear their original name, Roche today has more than a dozen U.S. sites located in California, Colorado, Indiana, New Jersey and South Carolina, as well as in Puerto Rico. Roche has alliances and research and development agreements with numerous partners, including majority ownership interests in Genentech and Chugai. Roche's Pharmaceuticals Division offers a portfolio of leading medicines in therapeutic areas including cancer, HIV/AIDS, hepatitis C, transplantation, dermatology and influenza. Roche's Diagnostics Division supplies a wide array of innovative testing products and services to researchers, physicians, patients, hospitals and laboratories world-wide. For further information, please visit our worldwide and U.S. websites (Global:www.roche.com and U.S.:www.roche.us).
|
|
| |
| |
|
|
|