| |
Isatoribine, an agonist of TLR7, reduces plasma virus concentration in chronic hepatitis C infection
|
| |
| |
Authors conclude: ".... This proof-of-concept study with intravenously administered isatoribine establishes that a TLR7 agonist can elicit anti-HCV effects in a short-duration study.... And may represent an important new approach for treatment of chronic HCV infection and may increase motivation for additional exploration of TLR ligands in a range of other disorders."
Hepatology
Volume 42, Issue 3, Pages 724-731
Published Online: 22 Aug 2005
Yves Horsmans 1, Thomas Berg 2, Jean-Pierre Desager 1, Tobias Mueller 2, Eckart Schott 2, Simon P. Fletcher 3, Kevin R. Steffy 3, Lisa A. Bauman 3, Bradley M. Kerr 3, Devron R. Averett 3 *
1Cliniques Universitaires Saint-Luc, Université Catholique de Louvain, Unit de Pharmacologie Clinique, Brussels, Belgium
2Universitätsklinikum Charité, Humboldt-Unversität zu Berlin, Campus Virchow, Hepatologie und Gastroenterologie, Berlin, Germany
3Anadys Pharmaceuticals, Inc., San Diego, CA
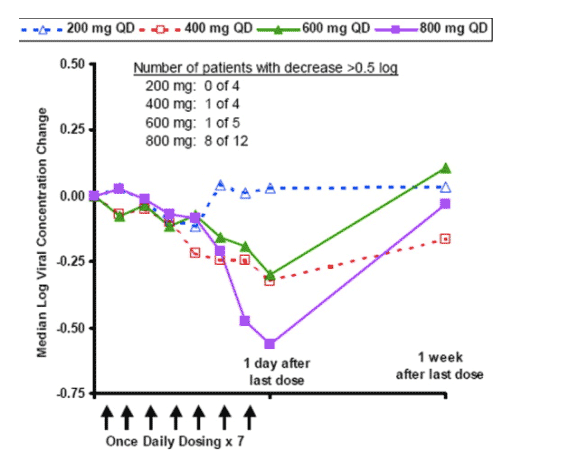
Figure 1. Median logarithmic change in hepatitis C virus (HCV) RNA in plasma (log viral concentration) from pre-treatment baseline by dose group in patients chronically infected with HCV who received treatment with intravenous isatoribine once daily (QD) for 7 days. The log viral concentration change from baseline to end-of-treatment in the 800 mg QD dose group was significant (Wilcoxon signed rank test, P = .001).
Toll-like receptors (TLRs) are now established to be a family of pathogen recognition receptors that initiate the innate immune response.[10-12] Stimulation of TLRs directly or indirectly causes the release of multiple cytokines, including type 1 and type 2 interferons, the induction of pathways and enzymes that destroy intracellular pathogens, the activation of a variety of cellular responses, and the priming of the adaptive response by activating immature dendritic cells and inducing their differentiation into professional antigen-presenting cells. At least 11 different TLR genes have been identified in humans.[13] Among these, TLR7 is of particular interest from a medicinal chemistry standpoint because small molecule ligands for this receptor have been identified,[14-17] including the guanosine analog isatoribine (also referred to in the literature as 7-thia-8-oxoguanosine).[14] In preclinical studies, isatoribine does not exhibit direct antiviral activity or significant cytotoxicity in vitro; however, through stimulation of innate immunity, isatoribine does prevent or reverse otherwise lethal viral infections in various acute infection models in mice.[18-21] Based on this preclinical profile, and in view of the need for new anti-HCV therapies, we performed a clinical proof-of-concept study with isatoribine to determine whether treatment with a TLR7 agonist can achieve a demonstrable antiviral effect at a well-tolerated dose in patients chronically infected with HCV.
ABSTRACT
Immune-based therapy is the mainstay treatment for chronic hepatitis C virus (HCV) infection but causes multiple side effects and achieves durable viral clearance in only approximately 50% of patients. Most new investigational anti-HCV compounds are direct-acting antivirals for which durability of response and risk of viral mutations and resistance are not yet known. Therefore, continuing discovery and development of new immune-based treatments is desirable. Toll-like receptors (TLRs) are pathogen recognition receptors that initiate the innate immune response. The responsiveness of HCV or other ongoing chronic systemic infections to treatment with a selective TLR agonist has not been reported. Isatoribine is a selective agonist of TLR7.
In a proof-of-concept study, we found that once-daily 7-day treatment with intravenous isatoribine 800 mg caused a significant (P = .001) reduction of plasma HCV RNA (mean, -0.76; range, -2.85 to +0.21 log10 units) in otherwise untreated patients (n = 12) who were chronically infected with HCV. Viral load reduction occurred in patients infected with genotype 1 as well as non-genotype 1 HCV.
The patient population was heterogeneous, but the study was not sized or powered to stratify and analyze various demographic, disease, and viral predictors of response to therapy. Nevertheless, viral concentration reductions were observed in patients of all tested viral genotypes, with the largest reductions of more than 2 log10 units found for both genotype 1 (a patient in the 400 mg twice daily dose group) and genotype 3 (a patient in the 800 mg once daily dose group). The 6 patients in this study who showed viral concentration decreases of approximately 1 log10 unit or more had pretreatment plasma HCV RNA values that ranged from low (32,000 IU/mL) to high (3.4 million IU/mL) by conventional HCV baseline categorization.
The reduction of viral load was correlated with induction of markers of a heightened immune antiviral state, including 2-, 5- oligoadenylate synthetase levels in whole blood. This treatment was well tolerated, with a low frequency of mild to moderate adverse events.
In conclusion, systemic administration of the selective TLR7 agonist isatoribine resulted in dose-dependent changes in immunologic biomarkers and a statistically significant antiviral effect with relatively few and mild side effects.
Safety Results.
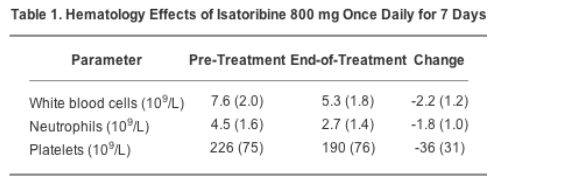
Adverse events during treatment with isatoribine were all of mild or moderate grade. No patients withdrew from the study because of adverse events or clinical laboratory abnormalities. The most frequent adverse events were insomnia (4 of 32 patients), joint pain (4 of 32), headache (4 of 32), and asthenia (4 of 32), of which only joint pain appeared possibly related to the dose level of isatoribine (3 of the 4 cases occurred in the highest once-daily dose group). The only notable laboratory or hematology changes were modestly decreased white blood cell, neutrophil, and platelet counts, as shown in Table 1 for the expanded 800 mg once-daily dose group. These hematology effects are reminiscent of changes observed during treatment with interferon-alpha-based therapy. However, unlike directly administered cytokine therapy, which often causes moderate to severe flu-like symptoms, the TLR7 agonist isatoribine elicited few reports of fever (2 of 32 patients), nausea, vomiting, and chills (1 patient), or influenza-like symptoms (2 patients), and these few were of mild or moderate grade.
Discussion
Anti-HCV activity in this proof-of-concept study was associated with induced expression of OAS and ISG15, and no patients in this study achieved a discernible viral concentration reduction in the absence of evidence for enhanced immunity. This association indicates that isatoribine must induce an immune response to achieve an anti-HCV effect, consistent with preclinical data.[18-21] Although OAS and viral concentrations showed little or no change after a single dose of 800 mg isatoribine, these biological markers were substantially changed both immediately before and 24 hours after the 7th daily dose. Circulating neopterin and IP10 were elevated after 6 days of once-daily administration of 800 mg isatoribine, providing additional evidence of immunological activation that is consistent with a TLR7 agonist mechanism. A few patients in this study showed increased expression of OAS and ISG15 with little or no effect on plasma viral concentration. This latter finding does not conflict with the TLR mechanism, but rather indicates only that other yet-to-be identified viral or host factors also have influence on the rate or depth of anti-viral response to a TLR7 agonist. One factor known to influence HCV response to other drug treatments is viral genotype.[1] In this study, anti-HCV effects of isatoribine were not obviously dependent on viral genotype. However, larger studies are needed to more fully evaluate this and other factors for impact on antiviral response to isatoribine.
Isatoribine, a guanosine analog, has some chemical structure features in common with the ribonucleoside ribavirin, a component of current standard-of-care therapy for HCV infection. The exact mechanism by which ribavirin contributes to anti-HCV activity is not fully understood, and may involve both immune-modulating and direct antiviral actions.[33][34] However, at in vitro concentrations up to approximately 200 mol/L (several-fold higher than plasma concentrations achieved in patients), ribavirin lacks the TLR7-activating properties of isatoribine,[14][35] and so the mechanism of anti-HCV activity by isatoribine is likely distinct from that of ribavirin.
Numerous nucleoside analogs with antiviral properties are historically documented, and many of these are in current medical use.[36] Antiviral activities for such compounds are most often mediated through a direct-acting mechanism in which a derived nucleotide binds to a virally encoded receptor or enzyme. Although isatoribine is structurally related to many direct-acting antiviral nucleoside analogs, the TLR7-activating mechanism of isatoribine is entirely different, eliciting antiviral activity through stimulation of a host receptor rather than through inhibition of a viral receptor. Consequently, future longer-duration studies with isatoribine or a related TLR7 agonist may reveal a much lower risk of viral mutations and drug resistance than is typically observed with other nucleoside antiviral agents.
Individual cytokines can be exploited to heighten the immune response and thereby induce an antiviral state. The use of interferon-alpha-based therapy for treatment for HCV infection is an example of this approach. However, direct cytokine administration frequently causes multiple side effects that are difficult for patients to tolerate.[3][37][38] We hypothesized that broader stimulation of innate immunity through TLR stimulation may avoid excessive exposure to any single cytokine and therefore may achieve antiviral benefits of heightened immune response with fewer and more tolerable side effects than are generally experienced with direct administration of an individual cytokine. The current study with isatoribine provides some initial support to this hypothesis, in that a significant short-term antiviral effect was in fact achieved with relatively few and mild side effects, encouraging further testing of the concept by evaluation of cytokines, activation states of dendritic and other cells, and changes in adaptive immunity over longer treatment periods.
This proof-of-concept study with intravenously administered isatoribine establishes that a TLR7 agonist can elicit anti-HCV effects in a short-duration study. The HCV viral load decline in this study resembles that reported at the end of 1 week of treatment with interferon-based regimens,[22-25] although the detailed kinetics clearly differ. In any case, the therapeutic value of treatment using a TLR7 agonist can only be established through studies of longer duration. Treatment periods of 6 months to 1 year are the norm for current standard-of-care anti-HCV treatments. Treatment periods for a TLR7 agonist are likely to be of similar duration, which will require a formulation that is suitable for convenient oral administration. Pharmaceutical development of isatoribine has been limited by its poor oral bioavailability (unpublished data, Anadys Pharmaceuticals, San Diego, CA). However, unlike many other known TLR ligands,[13] isatoribine offers a tractable foundation for medicinal chemistry. This successful proof-of-concept study with intravenous isatoribine provides a rationale to develop successor molecules with improved characteristics for oral dosing to enable longer-term efficacy studies of isatoribine (or a related compound) in HCV-infected populations.
Theoretical medical uses of TLR engagement have been reviewed.[39-41] However, because of the novelty of this field, TLR modulation remains essentially unexplored as an approach to treat clinical viral infections or other human diseases. This study shows that administration of a TLR7 agonist can significantly increase pathogen clearance in a systemic infection. Imiquimod, another TLR7 agonist,[17] is useful for topical treatment of human genital papilloma infection and certain dermal cancers[42] but has not been reported to elicit antiviral responses in patients with systemic infections. The related molecule resiquimod, an agonist of both TLR7 and TLR8,[14] was reported in abstract to lack antiviral effects in HCV-infected patients,[43] perhaps because of tolerability difficulties that constrained dose level and frequency; a more extensive evaluation of resiquimod may provide better understanding of the relationship of benefit to adverse events observed with that drug. In contrast, the favorable findings we report here for a study of isatoribine indicate that well-tolerated selective TLR7 agonists may represent an important new approach for treatment of chronic HCV infection and may increase motivation for additional exploration of TLR ligands in a range of other disorders.
Results
Antiviral Effects.
Plasma HCV RNA decreased over the course of isatoribine treatment, with the larger changes generally occurring in patients who received the higher daily doses; this dose-response relationship was significant (P = .024) for the once-daily dose groups. These on-treatment viral concentration changes were much larger than can be explained by assay variability (see Patients and Methods for plasma HCV RNA assay performance details). Eight of 12 patients who received isatoribine 800 mg once daily for 7 days showed a plasma viral concentration decrease of more than 0.5 log10 units, including 4 patients with a decrease of approximately 1 log10 unit or more. The median and mean viral concentration change in these 12 patients was -0.56 and -0.76 log10 units, respectively, with a range of -2.85 to +0.21 log10 units. This decrease in viral concentration was statistically significant for the 800-mg once-daily dose group (P = .001), and the extent of decrease in this dose group after 7 days of dosing was comparable to that reported at 7 days after initiation of treatment with standard regimens of pegylated interferon alpha-2b plus ribavirin. Although group sizes for the alternative dosing frequencies were small, the 400 mg twice daily regimen yielded a range of anti-HCV effects that were similar to the 800 mg once daily dose group, whereas the 800 mg thrice weekly regimen had less effect. Plasma viral concentration declines generally reversed on cessation of treatment.
Immunological Effects.
Assays of whole blood RNA were performed to evaluate the effect of isatoribine on expression of two different interferon-response genes, namely ISG15 and the intracellular antiviral protein OAS. Other studies in HCV-infected patients have shown that interferon-based therapy is associated with upregulation of OAS and ISG15, although this does not necessarily predict long-term outcomes. In this study, we did not observe substantive increases in these markers after a single dose of isatoribine; however, dose-related increases in expression of both OAS and ISG15 were found at the end-of-treatment with isatoribine. The extent of OAS induction in the 800 mg once-daily dose group (median increase of 7.6 times relative to baseline) was comparable to that reported during the first week after start of treatment with pegylated interferon alpha-2b. The extents of OAS and ISG15 induction by isatoribine were closely correlated (correlation = 0.945, P <.001), but within a dose group there was considerable inter-patient variability in the extent of induction. Reduction of plasma HCV RNA was correlated to the induction in whole blood of ISG15 RNA (correlation = -0.626, P <.001) and OAS RNA (correlation = -0.604, P <.001). At approximately 1 week after cessation of isatoribine treatment (at the posttreatment follow-up visit), the levels of ISG15 and OAS RNA in blood had returned to pretreatment baseline levels.
ELISA assays showed that some circulating markers of immune induction were modestly increased after 800 mg once daily dosing. Median (range) trough plasma concentrations and average within-patient percent increase compared with pretreatment were as follows: interferon-alpha 0.9 (<0.6 to 3.5) versus <0.6 (<0.6 to 2.8) pg/mL, 41 ± 75% increase; IP10 492 (199 to 1104) versus 247 (143 to 867) pg/mL, 95 ± 120% increase; and neopterin 12.7 (4.8 to 19.4) versus 6.2 (4.5 to 7.5) nmol/L, 99 ± 69% increase. Concentration increases for interferon-alpha did not achieve statistical significance (P = .08), whereas increases for IP10 and neopterin were significant (P <.01). Neopterin is a known marker of immune activation produced by activated macrophages; IP-10 is a chemokine that signals through the CXCR3 receptor and selectively chemoattracts Th1 lymphocytes and monocytes. Both neopterin and IP10 are linked to endogenous interferon gamma production. The levels of neopterin observed at baseline and after 6 days of once- daily isatoribine dosing resemble the levels observed at baseline and after 6 weeks of interferon-alpha-2b dosing on a schedule of 3 million units thrice weekly.
Pharmacokinetic Results.
Plasma isatoribine concentrations were approximately proportional to the administered dose (systemic clearance approximately 30 L/h; Cmax approximately 10 g/mL at the highest dose of 800 mg). Inter-patient variability was low, with plasma isatoribine Cmax and area-under-the-curve values ranging 2.5 times or less from lowest to highest value among patients within a dose group. The elimination half-life of isatoribine from plasma was approximately 2 hours; consequently, little or no accumulation of isatoribine occurred during multiple dosing, and trough plasma drug concentrations at the end of a dosing interval were either very low or not measurable. At these same trough timepoints when isatoribine was very low or absent from plasma, OAS, ISG15, and HCV RNA were markedly altered at the end of the multiple-dose treatment period in many patients, especially in the 800 mg once-daily dose group (illustrated for OAS in Fig. 6). Thus, an antiviral state was induced with multiple transient exposures to isatoribine; continuous exposure was not required.
Patients and Methods
Clinical Study Approval.
This phase 1, open-label, dose-escalation clinical study of intravenous isatoribine in patients chronically infected with HCV (protocol ANA245-102) was reviewed and approved by the clinical study site Ethics Committee in Brussels, Belgium (Commission d'Éthique Biomédicale Hospitalo-Facultaire, Université Catholique de Louvain, Faculté de Médecine) and in Berlin, Germany (Ethikkommission, Charité - Universitätsmedizin Berlin, Campus Virchow-Klinikum), and by the German Health Authority (Bundesinstitut für Arzneimittel und Medizinprodukte, or BfArM).
Patients.
Each patient provided signed informed consent to participate in this investigational clinical study in accordance with institutional and regulatory guidelines. Major entry criteria were: (1) chronic HCV infection; (2) no cirrhosis or advanced or decompensated liver disease; (3) no anti-HCV or immunomodulatory therapy in the preceding 90 days; (4) no co-infection with human immunodeficiency virus or hepatitis B virus; (5) no medical conditions or concomitant drug use that would interfere with study objectives. Participation in this dose escalation study was offered to sequential patients meeting entry criteria. The study population was predominantly male (20 of 32 patients), white (26 of 32), and middle-aged (average age of 45 years; range, 19-62 years). Patients were naïve to interferon-alpha-based therapy (17 of 32) or had partially responded to or relapsed from such therapy. Most patients were infected with HCV genotype 1 (23 of 32, or 72% of patients). HCV genotype 3 (3 patients) and genotype 4 (6 patients) accounted for the remainder of patients. Median (range) baseline plasma HCV RNA values (IU/mL) were 727,000 (32,000-4,000,000), which when transformed to log10 values were 5.9 (4.5-6.6). Median (range) baseline serum alanine aminotransferase values were 54 (20-116) IU/L.
Dosing and Safety Assessments.
Each dose of isatoribine was administered as a 60- to 80-minute intravenous infusion in sterile normal saline. Most patients in this study (25 of 32) received isatoribine once daily for 7 days at the following dose levels: 200 mg once daily (n = 4 patients), 400 mg once daily (n = 4), 600 mg once daily (n = 5), and 800 mg once daily (expanded cohort, n = 12). Alternative dose frequencies were also evaluated in 2 other dose groups: 400 mg twice daily for 7 days (n = 3 patients) and 800 mg thrice weekly (Monday, Wednesday, Friday) for 2 weeks (n = 4 patients). For the twice-daily and once-daily dose groups, patients were queried daily for adverse events from pretreatment (day -1) through 1 day after the final dose, and at the follow-up visit 1 week post-dosing. Hematology and vital signs were assessed every 1 to 2 days from pretreatment through 1 day after the final dose, and again at follow-up; other routine clinical laboratory assessments were assessed at pretreatment, mid-way through the treatment period, 1 day after the final dose, and at follow-up. Physical examinations were performed and electrocardiograms assessed at the beginning and end of treatment and at follow-up. For the thrice-weekly dose group, a similar set and quantity of safety assessments were performed, distributed over a treatment period of 12 days.
Pharmacodynamic and Pharmacokinetic Sampling.
Plasma samples for HCV RNA measurement were collected from all patients on day -1 and at predose on day 1 (first day of treatment), at predose on selected days during treatment, 1 day after the last dose of isatoribine (end-of-treatment), and at the follow-up visit approximately 1 week after the last dose of isatoribine. Serial plasma samples through 24 hours post-dose were collected for measurement of plasma isatoribine on the first and last day of isatoribine treatment. Whole blood samples were collected (PAXgene collection tubes, Qiagen, Valencia, CA) for isolation of total cellular RNA to determine the effect of isatoribine on host immune gene expression. RNA was analyzed by RNase protection assay or branched DNA (bDNA) assay at predose on day 1 (pretreatment), at predose on the last day of treatment, 1 day after the last day of treatment, and at the follow-up visit approximately 1 week after the last dose of isatoribine.
Plasma HCV RNA Assay.
A commercial bDNA assay (Versant HCV RNA 3.0 Assay, Bayer Diagnostics, Tarrytown, NY) was used to quantify HCV RNA in plasma. The dynamic range for this assay was approximately 6.2 _ 102 to 7.7 _ 106 IU/mL, which expressed as log10 transformed IU/mL values was approximately 2.8 to 6.9. All plasma samples from a given patient were assayed in a single batch run; samples at key time-points (baseline, end-of-treatment) were assayed in duplicate or triplicate within the batch run. Among 422 duplicate assays in this study, the median and maximum absolute difference between assay pairs was 0.03 and 0.41 log10 units, respectively, with most of the duplicate assays (397 of 422, or 94%) having differences of less than 0.10 log10 units.
Host Immune Gene Expression Assays.
A custom multi-template probe set for RNase protection assays incorporating the housekeeping genes L32 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was obtained from PharMingen (San Diego, CA). Additionally, the p40 (small) 25-oligoadenylate synthetase (OAS) template was amplified from the 25-OAS 1 human complementary DNA (Orbigen, San Diego, CA) and sub-cloned into pUC19 (New England Biolabs, Ipswich, MA). After in vitro transcription, this gave a probe of 228/220 nt (unprotected/protected). Similarly, the template for interferon stimulated gene 15 (ISG15) was amplified from isatoribine-stimulated peripheral blood mononuclear cell total cellular RNA and was then also sub-cloned into pUC19. This gave a probe of 373/360 nt (unprotected/protected). The identity of the p40 OAS and ISG15 probe templates was confirmed by sequencing the clones in their entirety. The in vitro probe transcription and subsequent RNase protection assays of RNA isolated from whole blood were performed using the PharMingen RiboQuant kit essentially as described in the manufacturer's protocol. Total cellular RNA was purified from 7.5 to 12.5 mL samples of whole blood by PAXgene purification kits (PreAnalytix, Qiagen). Relative levels of the protected RNAs were determined by phosphorimager densitometry (BioRad Molecular Imager FX, Hercules, CA) and were normalized to levels of L32 and GAPDH (housekeeping genes). For 1 isatoribine dose group only (400 mg twice daily), OAS and ISG15 measurements (normalized to GAPDH) were determined by bDNA rather than RNase protection assay because of a low quantity of RNA isolated from some of the whole blood samples in this dose group. Based on provided sequences of ISG15 [NM_005101] and p40 OAS [NM_002534], Genospectra (Fremont, CA) designed and synthesized probes to these analytes. Whole blood RNA from the 400 mg twice daily dose group was then analyzed for OAS, ISG15, and GAPDH by bDNA essentially as described in the Genospectra protocol.
Plasma Cytokine Assays.
Validated ELISA assays were used to determine levels of interferon-alpha, neopterin, and the chemokine IP10 in heparinized plasma samples from the 12 patients in the 800 mg once daily dose group. Other dose groups were not assessed. The interferon-alpha kit (RPN2789) was from Amersham Biosciences (Piscataway, NJ) and had an assay linear range of 0.6 to 20 pg/mL. The neopterin kit (014-HD-99.1) was from Alpco (Windham, NH) and had an assay linear range of 2.0 to 250 nmol/L. R&D Systems (Minneapolis, MN) supplied the kit for IP10 (DIP-100), which had a linear range of 7.8 to 500 pg/mL.
Plasma Isatoribine Assay.
A validated high-pressure liquid chromatography/MS-MS method was used to assay plasma isatoribine for pharmacokinetic purposes. The assay linear range was 3 to 3,000 ng/mL. Samples with concentrations higher than 3,000 ng/mL were reassayed after dilution with blank plasma, with final reported concentrations adjusted for dilution as appropriate. Based on calibrator and quality control samples, inter-day assay precision was within 11.1% and accuracy was within 5.2% of nominal concentration.
Data Analysis and Statistics.
For each patient, pretreatment baseline plasma HCV RNA value was calculated as the average of log10 transformed IU/mL values on day -1 and 1 (at predose). The effect of isatoribine treatment on plasma HCV RNA (change in log10 transformed IU/mL values) was determined for each patient by taking the difference between the end-of-treatment value (1 day after the last isatoribine dose) and pretreatment baseline value. The significance of changes in log10-transformed plasma HCV RNA values from pretreatment baseline to the end-of-treatment, within each dose group, were assessed using the Wilcoxon signed rank test. The Jonckheere-Terpstra test was used to test for a dose response in viral load change across the four once-daily dose groups. OAS and ISG15 values (relative to L32 and GAPDH) at selected times after initiation of isatoribine treatment were normalized to the patient's baseline value, which was estimated as the average of the pretreatment day 1 value and the 1-week posttreatment follow-up value (by which time treatment effect had dissipated). OAS and ISG15 at end-of-treatment in each patient were estimated by averaging the pre-dose value on the last day of dosing and the value at 1 day after the last dose. The relationships between OAS and ISG15 values, and between OAS or ISG15 and HCV viral load declines, were assessed using the Spearman rank correlation coefficient. Pre-dose plasma concentrations of interferon-alpha, IP10, and neopterin were compared between the first and last (seventh) doses using a paired t test. Hematology values at pre-treatment and end-of-treatment were compared by paired t test. Pharmacokinetic parameters for plasma isatoribine were calculated by standard noncompartmental methods.
|
|
| |
| |
|
|
|