| |
Noninvasive Fibrosis Test Performs Well
|
| |
| |
"Value of two noninvasive methods to detect progression of fibrosis among HCV carriers with normal aminotransferases"
Hepatology
October 2005
Cosimo Colletta 1, Carlo Smirne 2, Carlo Fabris 3, Pierluigi Toniutto 3, Rachele Rapetti 2, Rosalba Minisini 2, Mario Pirisi 2 *
1COQ, Madonna del Popolo Hospital, Omegna, Italy
2Department of Medical Sciences, University of Eastern Piedmont A. Avogadro, Novara, Italy
3DPMSC, University of Udine, Udine, Italy
The study authors summarize:
"....The current study documents that, in the evaluation of HCV carriers with Normal ALT, Fibroscan has a far better correlation with liver biopsy than FibroTest. Obviously, this finding does not prove that Fibroscan is a perfect tool to estimate liver fibrosis, only that it matches perfectly with sampling errors inherent with liver biopsies.... Fibroscan is superior to the Fibrotest...."
The EDITORIAL (see at end of report) says:
"....If the ultrasound elastographic findings of this study are indeed verified, would this herald the end of the liver biopsy for fibrosis assessment? Although certainly promising, it may be premature to jump to that conclusion. A liver biopsy provides other useful information in addition to fibrosis determination. It is used to diagnose, grade and stage disease.[19] Fibroscan was developed as a means to stage disease. It is unable to determine the cause of a liver disease or to distinguish subtle diagnostic differences such as nonalcoholic steatohepatitis (NASH) from nonalcoholic fatty liver disease (NAFLD), diagnose rejection or graft versus host disease. Nor can Fibroscan grade disease activity (amount of necroinflammation or severity of injury) as in NASH or primary biliary cirrhosis... from this study were quite striking......the high rate of liver fibrosis progression in patients with persistently normal ALT levels."
Abstract
The course of hepatitis C virus (HCV) infection carriers with normal/near-normal aminotransferases (NALT) is usually mild; however, in a few, fibrosis progression occurs. We aimed to verify whether monitoring by liver biopsy might be replaced by noninvasive methods and to identify factors associated with fibrosis progression in patients with persistently normal alanine aminotransferases.
We studied 40 untreated HCV-RNA-positive subjects (22 male; median age, 44 years), who underwent two liver biopsies, with a median interval of 78.5 months, during which alanine aminotransferase concentrations (median number of determinations: 12) never exceeded 1.2 times the upper normal limit. Within 9 months from the second biopsy, they were tested by the shear elasticity probe (Fibroscan) and the artificial intelligence algorithm FibroTest. METAVIR fibrosis scores were analyzed in relationship to demographic, clinical, and viral parameters. Weighted kappa analysis was used to verify whether the results of noninvasive methods agreed with histology.
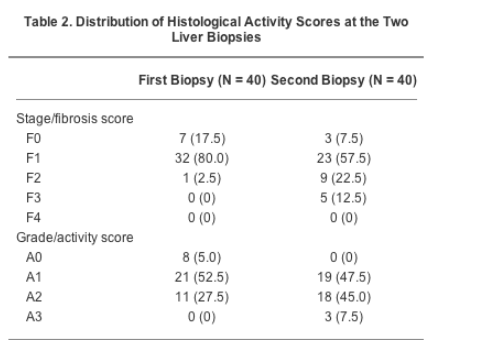
Significant fibrosis (F2), present at the first biopsy in only one patient (2.5%), was observed at the second biopsy in 14 patients (35%). At multivariate analysis, excess alcohol consumption in the past (>20 g/d; P = .017) and viral load (>8.0 × 106 copies/mL; P = .021) were independent predictors of progression. In identifying patients with significant fibrosis, inter-rater agreement was excellent for Fibroscan (weighted kappa = 1.0), and poor for FibroTest (weighted kappa = -0.041).
In conclusion, among HCV carriers with NALT, Fibroscan is superior to the FibroTest in the noninvasive identification of fibrosis, for which excess alcohol consumption in the past and high viral load represent risk factors.
Accuracy and Inter-rater Agreement of Noninvasive Methods of Fibrosis Detection
Both FibroTest and Fibroscan were performed within a maximum of 9 months from the second biopsy (median, 6 months). In Fig. 1, individual FibroTest results (upper panel) and liver stiffness values by Fibroscan (lower panel) are plotted against METAVIR fibrosis scores at the second liver biopsy. In identifying patients with significant fibrosis (METAVIR F2 or F3), choosing the previously reported cutoff values of 8.7 and 9.6 kPa,[9] the agreement between Fibroscan and liver biopsy was excellent, with perfect concordance of results (weighted kappa = 1.0). In contrast, the agreement between FibroTest and METAVIR to identify patients with negligible fibrosis (METAVIR F0-F1), choosing the cutoff value (0.31) suggested by the FibroTest manufacturer, was poor (weighted kappa = -0.041). Specifically, 26 (100%) of 26 of the patients for whom antiviral treatment is likely to be postponed (i.e., those with a METAVIR fibrosis scores F0-F1) could have been spared a liver biopsy by performing 1-dimensional transient elastography, in comparison with only 10 (38%) of 26 by FibroTest. Furthermore, without the need for a liver biopsy, 14 (100%) of 14 of the patients eligible for antiviral treatment (i.e., those with a METAVIR fibrosis score F2 or greater) could have been identified by means of the Fibroscan, in comparison with 9 (64%) of 14 identifiable by means of the FibroTest: Fibroscan would have also provided a correct estimate of patients with extensive fibrosis (METAVIR F3). Table 4 shows sensitivity, specificity, and accuracy for both FibroTest and Fibroscan in the study population.
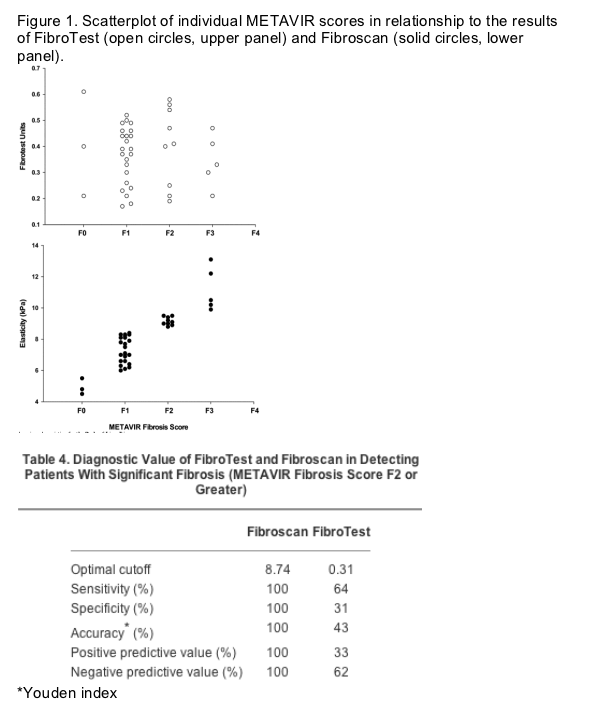
Accelerated Progression of fibrosis Observed in Study Patients
The median activity scores were A1 at the first, and A2 at the second biopsy, respectively, whereas the median fibrosis score was F1 at both biopsies. However, at the second biopsy, fibrosis was found to be progressed to a significant degree (METAVIR fibrosis score F2 or greater) in approximately one third of patients. In detail, of the 7 patients with fibrosis score F0 at the first biopsy, 2 remained F0 and 5 progressed to F1. Of the 32 patients with fibrosis score F1 at the first biopsy, 1 regressed to F0, 18 remained F1, 9 progressed to F2, and 4 to F3. The single patient with F2 at the first biopsy progressed to F3. Moreover, significantly more necroinflammatory activity (P < .001) and fibrosis (P < .001) were observed in the second biopsy, in comparison with the first.
Discussion
The current study documents that, in the evaluation of HCV carriers with NALT, Fibroscan has a far better correlation with liver biopsy than FibroTest. Obviously, this finding does not prove that Fibroscan is a perfect tool to estimate liver fibrosis, only that it matches perfectly with sampling errors inherent with liver biopsies.
Estimation of liver fibrosis by FibroTest and Fibroscan stems from very different premises. The 5 parameters used to perform FibroTest were chosen by logistic regression operated on a selection of basic serum biochemical markers, having histological staging as the independent variable.[7] The original database for this study included 339 patients with biopsy-proven hepatitis C, 40% of whom with significant fibrosis. Mean ALT values were thrice the upper limit of the reference range for males, and only 13% of the studied patients had ALT within the normal range. Because biochemical markers were measured once, on the day of biopsy, and during the course of HCV infection ALT fluctuate widely, it is likely that only a few, if any, were true HCV carriers with NALT. Besides, in a subsequent FibroTest prospective validation study, participants needed documented elevated serum ALT levels (at least 1.5 times the upper limit of normal) on three occasions within 6 months before enrollment.[8] It is conceivable that these specifics might affect FibroTest performance when applied to HCV carriers with NALT, and that, for FibroTest to be a more reliable method to assess fibrosis in this category of patients, the database should be expanded to include more subjects with their characteristics.
In contrast, Fibroscan infers liver fibrosis by direct measurement of liver elasticity, with which fibrosis is highly correlated. Though intuitively informative in patients with chronic hepatitis, measurement of liver elasticity has been so far precluded by technical limitations and costs. With Fibroscan, however, a transmitted elastic wave can be temporally separated from reflected elastic waves, making the technique comparatively less sensitive to those boundary conditions (including body fat) that would induce artefacts. The acquisition time (typically less than 100 ms) enables measurements to be made on a moving organ such as the liver, and allows a painless, rapid examination with reproducible results,[10] at costs highly competitive with respect to biopsy. When applied to patients with biopsy-proven hepatitis C, Fibroscan proved to be a reliable tool to detect significant or extensive fibrosis, with optimal stiffness cutoff values of 8.7 and 9.6 kPa for F 2 and F 3, respectively.[9] Using exactly the same cutoff values, the current study documents even better sensitivity and specificity values when Fibroscan is used in HCV carriers with NALT. The diagnostic accuracy might be overestimated in the current study, in which sample size is substantially smaller; however, one of the reasons why Fibroscan, in our hands, performed so well, might be that the patients we studied were leaner (having, on average, a BMI approximately 3 kg/m2 lower) than those studied by Ziol et al.,[9] none was obese, and just a few were overweight. Lack of body fat might have favored a more accurate measure of liver elasticity, because the fatty thoracic belt attenuates both elastic waves and ultrasound. This favorable situation will probably be applicable to future series including HCV carriers with NALT, who do tend to be lean, because BMI is one major determinant of ALT concentration.[16] Thus, Fibroscan might find in this special subgroup of HCV carriers (whose relevance is testified by its frequency, which means costs) its ideal field of application.
If the degree of diagnostic accuracy obtained here by Fibroscan will be confirmed in further studies, the technique is likely to be endorsed by many hepatologists, equally concerned about the low acceptance rate of liver biopsy by patients, who perceive it as an overly aggressive procedure,[17] the high costs both of biopsy and antiviral treatment,[6] and the risk of missing a diagnosis of advanced liver disease. Would it be possible to identify those patients who, because of an increased risk of developing fibrosis, will need to be monitored at shorter intervals? The current data indicate that for HCV carriers with NALT, a high viral load and a history of excess alcohol consumption are independent predictors of development of significant fibrosis. The relationship between the replication level of HCV and liver damage is controversial,[18][19] although patients with higher viremia might have more intense architectural changes and more severe progression of liver disease than those with lower levels of HCV replication.[20] A relationship between heavy alcohol intake and progression to cirrhosis in HCV carriers with NALT is more firmly established,[4] and avoidance of alcohol is recommended for all HCV carriers.[2] In fact, among them, those who consume alcohol, even within the limits suggested not to be exceeded by international drinking guidelines,[15] have increased viremia and hepatic fibrosis.[21] Although not detected by testing for carbohydrate-deficient transferrin, those patients in the current study who reported excess alcohol in the past might have been prone to persist in their drinking habits despite medical advice. Although alcohol consumption and viral load might be factors to take into consideration when estimating the risk of fibrosis progression, in clinical practice this will mean that caring physicians, rather than limiting monitoring to specific categories, will have to focus further on patient education. In the absence of a consensus opinion on the amount of alcohol that HCV carriers may be allowed to drink, the current data suggest that it may be safer for caring physicians to recommend these patients suspend any alcohol intake, whether they have elevated transaminases or not, and to reassess this issue at regular intervals.
Until recently, only expectant management and adoption of a healthy lifestyle were recommended for HCV carriers with NALT.[22-24] Indeed, in this peculiar subgroup, antiviral treatment was deemed less effective and potentially hazardous, in view of the risk of hepatitis flares.[25] Therefore, many authorities in the field believed that for HCV carriers with NALT there was no compelling indication to undergo a liver biopsy. This conservative approach is somewhat challenged nowadays. In fact, the risk of progression to fibrosis in these patients may not be negligible. We report data on a cohort of 40 HCV carriers with NALT, among whom 35% reached significant fibrosis. Although none of the patients had cirrhosis at the end of a median follow-up period of 7.5 years, fibrosis progression was observed in 19 (48%) of 40. In a recent U.S. study, the natural history of a similar cohort of HCV carriers with NALT was compared with the natural history of HCV carriers with elevated transaminases.[26] The proportion of HCV carriers with NALT who had significant fibrosis at the end of the follow-up (30%) did not differ from that observed here, but only 22.5% of the American patients had fibrosis progression.[26] This discrepancy is puzzling, because the population described in the current paper, having a lower proportion of drinkers, males, and overweight subjects, should have been at lower risk of fibrosis progression. The 2 cohorts might differ with regard to the duration of HCV infection: however, this variable is often difficult to assess (in the current study, a reliable estimate could be obtained in only 6 patients), and mean age is similar. One possible explanation for the higher rate of fibrosis progression in the current study is, of course, sampling error: when paired biopsy samples are analyzed blindly, a 1-stage change in the fibrosis score (-1 to +1), most likely reflecting sampling error or interpreter variability, is easily reported. In our study, all but 1 patient had F0 or F1 at baseline, so most changes in score in the second biopsy could only have occurred in 1 direction. An alternative (or additional) explanation, however, concerns the duration of follow-up, which in the current study was not shorter than 5 years (in comparison to only 2 years in the study by Hui et al.[26]).
Recently, the opinion that HCV carriers with NALT do not benefit from antiviral treatment has also been challenged. The results of a large, international, multi-center, randomized clinical trial have made it clear that, for HCV carriers with NALT, risks and benefits of an up-to-date combination antiviral regimen are similar to those acknowledged for patients with high ALT.[27] Whether antiviral treatment of all HCV carriers with NALT is also cost-effective is questionable, however, because mathematical models estimate that this option results, in patients with mild disease, in relatively high costs for quality-adjusted year of life gained.[28] Side effects of treatment are also a concern. Thus, highly motivated patients might undergo empiric treatment, particularly when infected by HCV-2 or HCV-3. For the management of the others, deferring therapy to a time when treatments are likely to have become more effective and better tolerated, while giving strong advice on lifestyle issues and monitoring fibrosis non-invasively, could be the most sensible choice.
In conclusion, among HCV carriers with NALT, Fibroscan is superior to the Fibrotest in the noninvasive identification of fibrosis, for which excess alcohol consumption in the past and high viral load represent risk factors.
Assessment of liver fibrosis: Palpate, poke or pulse?
Liver Biopsy or Noninvasive Fibrosis Test
EDITORIAL
Hepatology
October 2005
Marc G. Ghany 1 *§, Edward Doo 2
1Liver Diseases Branch, Division of Digestive Diseases and Nutrition, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD
2Liver Disease Research Branch, Division of Digestive Diseases and Nutrition, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD
Article Text
Liver fibrosis is the cardinal feature of cumulative injury from chronic liver disease and is responsible for most of the terminal clinical features. Assessing fibrosis accurately is vital to the management of patients with chronic liver disease. Quantifying the extent of liver fibrosis is an important factor in the decision to recommend treatment, to assess treatment response and to decide upon when to begin screening for liver cancer and varices. Historically, advanced fibrosis or cirrhosis was diagnosed on clinical grounds by examining the patient for stigmata of chronic liver disease and palpating a shrunken, firm, nodular liver. This is a relatively insensitive manner to diagnose cirrhosis and certainly less so for minor degrees of fibrosis. The advent of liver biopsy allowed the earlier stages of fibrosis to be diagnosed, which facilitated institution of early therapy to prevent disease progression. Thus, for the past 6 decades a liver biopsy has been the accepted gold standard for assessing fibrosis. However, liver biopsy is not without drawbacks. It has low patient acceptance because it is invasive and associated with some discomfort and risk to the patient.[1] Moreover, sampling error of at least 24% is reported usually because of specimen fragmentation or inadequate length.[2-4] Interpretation of the biopsy is also subject to diagnostic inconsistencies due to inter- and intra-observer error.[5][6] As such, there has been a flurry of interest in developing new, noninvasive modalities to assess fibrosis such as biochemical markers, biomarkers, and new imaging techniques. These approaches have included surrogate markers of fibrosis consisting of readily available biochemical tests such as the Forns index,[7] AST to platelet ratio index (APRI),[8] and the commercially available FibroTest.[9] Newer advances such as serum protein glycomics[10] and proteomics[11] may also have a role in fibrosis assessment, especially when used in conjunction with existing methods such as the FibroTest.
The latest technological advance in fibrosis assessment is the Fibroscan, a specially adapted ultrasound machine that uses the principle of one-dimensional transient elastography to measure liver stiffness.[12] A special probe is used to both generate and measure the velocity of a low amplitude, low frequency vibration that is transmitted from the skin to the liver tissue. Pulse-echo ultrasound is used to measure the velocity of the shear wave through the liver tissue. The velocity of the shear wave is directly proportional to the stiffness of the tissue and thus indirectly measures extent of fibrosis: the stiffer the tissue the faster the shear wave. The machine reports a value in kilopascals (kPa), which can then be extrapolated to a fibrosis score.
Preliminary studies have shown the Fibroscan to be a useful device for assessing fibrosis. Two initial studies in patients with chronic hepatitis C, including all stages of fibrosis, reported good accuracy for predicting Metavir stage F2 to F4 with ROC values ranging from 0.79 (F2) to 0.97 (F4).[13][14] However, there was considerable overlap in the range of liver stiffness measurements among metavir stages which may have been related to liver biopsy issues such as small sample size (median biopsy length in both studies ranged from 17 to 18 mm), and differences in degree of necroinflammation and steatosis. Fibroscan performed better in separating cirrhosis (F4) from lesser degrees of fibrosis and in samples with longer biopsies.
In this issue of HEPATOLOGY, Colletta and colleagues from Italy report upon the correlation of the Fibroscan and FibroTest with liver biopsy results in a cohort of patients with chronic hepatitis C with persistently normal serum alanine aminotransferase (ALT) levels.[15] Forty untreated HCV RNA-positive patients with well-documented ALT levels less than 1.2 × upper limit of normal (ULN) underwent two liver biopsies separated by a median interval of 78.5 months. All patients were tested once by Fibroscan and FibroTest within a period of 9 months after the second biopsy. Significant fibrosis defined as Metavir stage F2 was present in 1 patient (2.5%) at initial liver biopsy and in 14 patients (35%) at second biopsy. Fibrosis progressed by one or more stages in approximately half of the patients. Only 1 patient had regression in fibrosis stage. Using the previously reported cutoffs of 8.7 and 9.6 kPa for Metavir fibrosis stages 2 and 3 respectively, the agreement between Fibroscan and liver biopsy assessment of fibrosis was perfect with a weighted kappa of 1.0. In contrast, the concordance between FibroTest and liver biopsy was poor with a weighted kappa of -0.041. Excess alcohol consumption (>20 g/day) and high viral load (>8 × 106 copies /mL) were identified by logistic multivariate regression to predict fibrosis progression in this cohort.
Two results from this study were quite striking. First was the high rate of liver fibrosis progression in patients with persistently normal ALT levels and the second was the remarkable capability of ultrasound elastography to distinguish the full spectrum of histopathological fibrosis stages. Both findings are in stark contrast to previously published data regarding the natural history of hepatitis C fibrosis accumulation with minimal transaminase activity[16][17] and with the ability of Fibroscan to clearly separate distinct fibrosis stages.[13][14][18] The perfect agreement between liver biopsy and Fibroscan raises the possibility that Fibroscan is influenced by similar errors inherent to liver biopsy such as the effect of subcapsular fibrosis or that both sample predominantly the right lobe of the liver. Alternatively, if the factors that contribute to errors of liver biopsy presumably differ from those of Fibroscan then it would suggest that the sample size of the study was not large enough to discern this difference.
If the ultrasound elastographic findings of this study are indeed verified, would this herald the end of the liver biopsy for fibrosis assessment? Although certainly promising, it may be premature to jump to that conclusion. A liver biopsy provides other useful information in addition to fibrosis determination. It is used to diagnose, grade and stage disease.[19] Fibroscan was developed as a means to stage disease. It is unable to determine the cause of a liver disease or to distinguish subtle diagnostic differences such as nonalcoholic steatohepatitis (NASH) from nonalcoholic fatty liver disease (NAFLD), diagnose rejection or graft versus host disease. Nor can Fibroscan grade disease activity (amount of necroinflammation or severity of injury) as in NASH or primary biliary cirrhosis. Thus, it will not replace ALT but might supplant liver biopsy to assess progression of disease toward cirrhosis.
As with any new diagnostic test, considerable assessment and validation is needed prior to introduction into general clinical practice. Preclinical evaluation should address issues such as inter and intra-observer test concordance and precision. An appropriate evaluation would be prospective and blinded in design and include the full spectrum of patients (different ages, races, body mass indices [BMIs]), variety of chronic liver diseases such as viral hepatitis, NASH, cholestatic and metabolic diseases along with accurate pathologic evaluations (liver biopsies of adequate size to stage fibrosis) using reasonable clinical endpoints such as progression of fibrosis by one point. The test should also be validated using other well-studied scoring systems such as Ishak.[20] In this way, the generated cutoff values determined from such a study would place ultrasound elastography on solid ground for use in practice. Currently, elastographic study results are derived mostly from selected patients with chronic hepatitis C.
Even with appropriate evaluation of the test dynamics, additional issues still need to be tackled in order to broadly apply this technology. Several technical limitations preclude the use of ultrasound elastography in approximately 5% to 8% of patients.[13][14][21] The principal reason for this failure is obesity. A much higher failure rate would be expected in the United States given the higher BMI of the general population.[22] However, this challenge is being addressed with further developments in ultrasonic probe technology. Additional limitations include a narrow intercostal space and ascites. However, ascites is typically indicative of decompensated cirrhosis and an elastographic measurement is unlikely to alter the overall clinical management. The quality of the liver parenchyma and intrahepatic structures may also potentially affect the physics of elastography. Large vascular structures in the acquisition window have the potential for yielding false measurement readings. Similarly, although necroinflammation and steatosis were reported not to influence liver stiffness measurements this needs to be assessed in larger cohorts with different patterns of fibrosis (portal versus central) and more severe grades of steatosis.
Transient elastography is an appealing test to evaluate fibrosis because it can be performed rapidly, painlessly, with relative ease and is expected to have high patient acceptance. It would appear that Fibroscan has certain advantages over other diagnostic indices or predictive models based on laboratory tests in that it is completely noninvasive, provides a more direct measure of fibrosis, should not be affected by other disease states, and should theoretically be applicable to all chronic liver diseases. But, what does the future hold for this technology? Perhaps the best application of Fibroscan may be to monitor patients longitudinally after a baseline biopsy is performed or to postpone a biopsy until a certain threshold liver stiffness measurement is obtained. Furthermore, diagnostic accuracy might be improved by combining Fibroscan with other noninvasive modalities.[14] This could obviate the need for liver biopsies in a number of cases. A recent study suggested that Fibroscan might be useful for predicting clinical complications of end-stage liver disease. Foucher and colleagues correlated the elastographic measurements with clinical complications of cirrhosis.[21] They reported that the cutoff values of 27.5, 37.5, 49.1, 53.7, and 62.7 kPa based on ROC curves had a negative predictive value of >90% for the diagnosis of grade 2-3 esophageal varices, Child-Turcotte-Pugh B or C cirrhosis, past history of ascites, hepatocellular carcinoma and bleeding esophageal varices, respectively. Whether or not ultrasound elastography can be extended to risk stratify or screen for cirrhotic liver complications will need to be assessed in future long-term longitudinal studies. Lastly, ultrasound elastography may find a niche in monitoring response to treatment. Thus in clinical trials with blinded results, in specific diseases with well defined endpoints (such as long-term therapy of chronic hepatitis B) it would be important to determine whether Fibroscan can document response as well as or better than serial liver biopsies. For this reason Fibroscan should be incorporated now into studies of long-term therapy so that its efficacy can be evaluated.
Ultrasound elastography represents a significant step forward in attempting to overcome some of the deficiencies associated with needle liver biopsy. Ultimately, however, elastography is still limited by its sampling size, as it is only 100 times larger than liver biopsy and the fact that its diagnostic accuracy is linked to an imperfect gold standard, the liver biopsy. Therefore future research should continue to be pursued in the field of fibrosis assessment. Ideally, efforts should focus on imaging modalities that would provide a more global impression of fibrosis, on genetic markers that would allow risk stratification given the recent studies that have suggested a role for genetic polymorphisms in fibrosis progression,[23][24] and on diagnostic markers that correlate dynamically with extracellular matrix formation.
As antifibrotic therapies are brought forward to the clinical arena there will be an even greater need to assess fibrosis as a means of monitoring treatment effectiveness. The liver biopsy will still have a role to play in the diagnosis, grading, and assessment of chronic liver disease. However, completely noninvasive assessment of liver fibrosis may be only a pulse away.
|
|
| |
| |
|
|
|