| |
Editorial: 24 weeks PegIFN 2b for genotype 1/Low HCV-RNA
|
| |
| |
Efficacy of 24 weeks treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C infected with genotype 1 and low pretreatment viremia
Journal of Hepatology Jan 2006
Stefan Zeuzem
Department of Medicine, Saarland University Hospital, Homburg/Saar, Germany
Maria Butib, Peter Ferencic, Jan Sperld, Yves Horsmanse, Janusz Cianciaraf, Endre Ibranyig, Ola Weilandh, Stephanie Novielloi, Clifford Brassi, Janice Albrechti
b S. Hepatologia-Planta 9, Hospital Valle d'Hebron, Barcelona, Spain
c University Clinic of Vienna, Department of Internal Medicine IV, Vienna, Austria
d Institute for Clinical and Experimental Medicine, Department of Hepatogastroenterology, Videnska 1958/9, Prague, Czech Republic
e Hospital Saint Luc-UCL, Bruxelles, Belgium
f Warsaw Medical University, Warsaw, Poland
g St Lazlo Hospital, Budapest, Hungary
h Karolinska University Hospital Huddinge, Department of Infectious Diseases, Stockholm, Sweden
i Schering-Plough Research Institute, Kenilworth, NJ, USA
ABSTRACT
Background/Aims: Previous studies using standard interferon and ribavirin combination therapy suggested that patients infected with HCV-1 and a low pretreatment HCV-RNA level can be treated for 24 weeks without compromising sustained virologic response rates. The aim of the present study was to investigate this schedule in the era of pegylated interferon-â┐ plus ribavirin.
Methods: Patients chronically infected with HCV-1 (n=235) and a screening viremia ≦600,000IU/mL (real-time PCR) were treated with peginterferon alfa-2b 1.5mg/kg subcutaneously once weekly plus ribavirin 800-1400mg/day based on body weight for 24 weeks.
Results: End-of-treatment and sustained virologic response rates were 80 and 50%, respectively. The 48-week historical control (Manns et al., Lancet 2001;358:958-65) had similar end-of-treatment (74%) but higher sustained virologic response rates (71%). This difference was due to a high virologic relapse rate after 24 weeks of therapy (37%) compared with the historical control (4%). A subset of patients who had undetectable serum HCV-RNA at treatment week 4, however, achieved similar sustained virologic response rate (89%) as in the control group (85%).
Conclusions: HCV-1 infected patients with a low baseline HCV-RNA concentration who become HCV-RNA negative at week 4 may be treated for 24 weeks without compromising sustained virologic response rates.
Further study details are following the Editorial immediately below.
EDITORIAL
Treating patients with HCV genotype 1 and low viraemia: More than meets the eye
Journal of Hepatology Jan 2006
Antonio Craxi, Calogero Camma
University of Palermo, Palermo, Italy
"......At the present time, the strength of the evidence presented by Zeuzem and co-workers is in our opinion not sufficient to generalize the applicability of this approach to optimization. While further randomized trials are in progress, subjects with genotype 1, a low viral load and an early drop of HCV-RNA under therapy may be treated with this short schedule if they have modest or no fibrosis and hence are unlikely to progress significantly should HCV relapse. Those with more advanced fibrosis are probably still better served by the conventional, more troublesome schedule of 48 weeks....."
When a drug or a combination of drugs is first assessed to evaluate its effectiveness in curing an illness, every effort is made in order to maximize efficacy while still keeping side effects at an acceptable level. It is hence not surprising that the pivotal phase III trials [1,2] which led in the early 2000s to the registration of pegylated alfa interferons (PEG IFNs) in combination with ribavirin for the treatment of naive patients with chronic hepatitis C have exploited the maximum permissible doses of PEG IFN evaluated in earlier monotherapy trials [3,4] over a 48-week treatment period. This duration of treatment had been shown to be the most effective with the combination of standard IFNs and ribavirin [5,6], and was in fact dictated by the need to demonstrate superiority of PEG over standard IFNs, already registered for a 48 week combination schedule. Both studies were concordant in showing a better efficacy of combination therapy with PEG IFNs, but confirmed that genotype 1 was still harder to eradicate than genotypes 2 and 3, and that subjects with severe fibrosis were less responsive to therapy. An important and concordant finding among these trials was that 12 weeks of treatment were enough to assess, by measuring the drop in viral load, whether sustained virological response was likely to be reached [7,8]. This early virological response (EVR) rule, allowing to stop ineffective therapy at an early stage, and save unnecessary treatment related morbidity could not be reliably used on standard IFN/ ribavirin regimens before 24 weeks of therapy [5,6]. A further step towards optimization was made by Hadziyannis et al. [9], who used a 4-arm design to assess the relevance of duration (24 vs. 48 weeks) and of ribavirin dose (800 vs. 1000-1200mg). This trial made it clear that genotype 1 patients should receive the more intensive approach with a longer treatment duration and higher doses of ribavirin, while genotypes 2 and 3 could do with shorter schedules and lower amounts of ribavirin. Optimization of treatment was thus initially addressed with the distinction between hard and easy to treat genotypes. Still, there was a long way to go towards true individualization of therapy, since the risk of overtreatment was still present for many patients not accurately classified by a genotype-based approach. It has long been known that viral load is also a major determinant of responsiveness to IFN-based therapy, whatever the schedule used for monotherapy [10] or for combination [11]. Problems with the reproducibility of quantitative measurements of HCV-RNA [12,13] have hampered a more widespread use of this predictor. Real-time PCR testing will probably be able to solve inconsistencies and allow clinicians to progress further into individualization of anti-HCV therapy.
The current 12 weeks EVR rule, when applied to genotype 1 patients, has a negative predictive value approaching 100% [7,8] and is hence able to solve the issue of overtreatment of nonresponders. It, however, leaves the field open to the possibility of overtreating some genotype 1 patients with a higher propensity for a sustained viral response, i.e. those with a pre-treatment low viral load. These subjects, representing in our experience 15-20% of all genotype 1 infected cases, could hypothetically be cured by shorter and/or less intensive treatment courses thus reducing costs and improving acceptance and tolerability.
In this issue of the Journal, Zeuzem et al. [14] test the hypothesis that patients infected with HCV 1 with low pretreatment HCV-RNA (≦600,000IU/ml at baseline) can receive PEG IFN alfa2b 1.5mg/kg weekly plus weight-based Ribavirin (800-1400mg daily) for 24 weeks and obtain the same rate of SVR as those treated for 48 weeks. The study is a single-arm, open-label historical control trial conducted in 43 European Centres between 2001 and 2004, in which 724 patients were screened and 237 enrolled. As a comparator, 38 comparable patients originally enrolled in the Manns' trial [1], who had received PEG IFN alfa2b 1.5mg/kg weekly plus Ribavirin üć10.6mg daily for 48 weeks, were re-analyzed. At first glance the results do not look encouraging as a whole since, after reaching end-of-treatment response (ETR) at a comparable rate (81% for 24 weeks and 74% for 48 weeks of treatment), sustained virological response (SVR) was obtained only by 50% in the 24 weeks as compared to 71% in the 48 weeks historical control group. This difference was due to the high rate of post-ETR virologic relapse (37%) on the short regimen.
The relevant information comes from the analysis of early virologic response (EVR) at 4, 12 and 24 weeks. A very high rate of SVR (89%) was in fact found in the subgroup of patients who were already HCV-RNA negative at week 4 (47% of all cases treated with the short course). Those with a slower response to therapy had instead a low rate of SVR (25% for patients becoming HCV-RNA negative at 12 weeks and 17% for those who cleared HCV-RNA only after 24 weeks). The pattern was seemingly different among patients treated for 48 weeks: SVR rates were 85, 93 and 67% for subjects clearing HCV-RNA at 4, 12 or 24 weeks, respectively. Thus low pretreatment viraemia associated to a precocious response to PEG IFN plus ribavirin identifies a subgroup of patients with genotype 1 in whom 24 weeks of treatment are sufficient to obtain the maximal SVR rate allowed by combination therapy. Optimization by avoiding overtreatment would thus become a reality in such cases. In fact, the registration of PEG IFN alfa2b has already been revised by EMEA to include this modality of use.
Issues arising during the phase of optimization of treatments are definitely more complex than the pure demonstration of safety and efficacy in registrative trials. Specifically, results generated by these trials in highly selected and motivated patients must be fine-tuned to fit into the complex array of real-life patients, who often have comorbidities or low compliance, and also keep account of demographic, social and financial constraints. It is of foremost importance, when interpreting the results of these studies, to make a clearcut distinction between evidence generated within RCTs by direct comparison of rigorously randomized groups and indirect inferences derived from the confrontation of non-randomized or 'historical controlüf groups, from subgroup analyses and from the application of a posteriori multivariate models or of mathematical situations. Only direct comparisons allow a reliable confrontation between groups which are fully comparable for all relevant features. At least two recent studies [15,16] have addressed the issue of optimization, in both cases for the easy-to-treat genotypes 2 and 3, using an appropriate design.
The Zeuzem study on genotype 1 patients with low viral load unfortunately has a number of limitations which potentially detract from its applicability. It has a low internal validity, due to the intrinsic weakness of a design which allows the use of a historical control group, derived from another study, performed more than 5 years ago. This control group is in itself a very small subgroup distilled from a large cohort (11.9%, i.e. 38 out of 348 genotype 1 patients receiving PEG IFN alfa2b 1.5mg/kg weekly plus Ribavirin 800mg daily in the original Mann's study). Although selection modalities are not stated, it may be inferred that patients were included in the historical control group only if: (a) they had received enough ribavirin in relation to body weight to allow for post-hoc adjustment of dosing at üć10.6mg daily; (b) treatment had been given as assigned for the entire 48 week period; (c) samples were available for testing at 4, 12 and 24 weeks. These requirements actually segregate a population of rather lean patients, with a body weight inferior to 75.5kg, with a high compliance to therapy and to follow-up, and with few or no treatment-related side effects forcing dose reduction or withdrawal. It comes as no surprise that in these 'idealüf subjects the SVR rate is 71%. By converse, it must be stressed that these patients were treated at a time when PEG IFNs were still experimental drugs: the degree of confidence of both the caregiver and the patient may well have been different from the current standard. Finally, the small size of subgroups in the EVR analysis (13 patients with EVR at 4 weeks, 15 at 12 weeks and 3 at 24 weeks) makes any comparison with the much larger 24 week group hard to substantiate.
Weight-based ribavirin was given over an extended range of choice (800-1400mg) in the intent to optimize the exposure to the drug. It is then odd to note that subjects on the higher doses of 1000-1200 tended to have more ribavirin-related adverse events than those on the lower dose. If a true optimization of ribavirin dosing is to be achieved, further studies taking into account individual variations in pharmacokinetics, possibly measuring plasma or whole blood levels are needed [17].
PEG IFN alfa2b was given at the standard recommended dosage of 1.5mg/kg weekly. While this dosage is clearly the most effective when given with low amounts of ribavirin (800mg), it is still debatable that such a dose is really needed to obtain response in patients with genotype 1. As a matter of fact, when PEG IFN alfa2b was used as monotherapy [3], 1.0mg/kg performed fractionally better than 1.5mg/kg. Recent results from an Italian multicenter trial in genotype 1 patients [18] show that an SVR of 41 can be reached by giving PEG IFN alfa2b approx. 1.0mg/kg weekly plus Ribavirin 1000-1200mg daily for 52 weeks. This response rate compares well with the 42% SVR reported by Manns [1] on 1.5mg/kg. In the Italian study patients who were HCV-RNA negative at 12 weeks had an ultimate SVR rate of 70%, thus confirming that EVR also applies to lower PEG IFN doses. Clearly, there is space for further optimization also in the reduction of the amount of IFN to be used.
Another matter of concern is the limited external validity, due to the modalities of inclusion within the study. A large number of cases (724) were screened for eligibility, but only 237 patients were enrolled. The low number of patients included at each site (on average 5.5 cases) represents a source of selection bias, since no data concerning the consecutivity of enrolment are given, and also a potential cause of heterogeneity in the actual handling of treatment.
It is not stated whether patients were admitted into the selection process before or after HCV-RNA quantification. If, as likely, quantification was one of the criteria to be assessed during screening, then an unknown proportion of the 454 who did not meet protocol criteria were left out because of an HCV-RNA value >600,000IU/ml. How was this tested? The issue may have major relevance in the context of a multicenter study, where 43 Centres have enrolled patients over a time span of 3 years. Since only a generic statement about the use of an rt-PCR is given by the Authors, it is possible that screening tests were actually done at each site with different methods and with a high potential for inconsistencies. Moreover, due to the spontaneous variations over time of HCV-RNA levels within a 0.5log range [19], it would have been worth assessing baseline HCV-RNA as a mean of values obtained at different points of observation in the months preceeding the start of therapy.
Definitely, the indications of this study cannot be extrapolated to subjects with advanced fibrosis or cirrhosis. Albeit individual data on the number of patients with cirrhosis are not given in Zeuzem's paper, the low mean Knodell score of 1.2 for fibrosis proves that advanced fibrosis was uncommon in the 24 weeks group. Consequently, as found by the Authors, it would be impossible to show upon univariate analysis any significant role of fibrosis on response in this cohort. In all megatrials [1,2,9] fibrosis has instead emerged as a significant predictor of resistance to the combination of PEG IFNs and ribavirin, although to a lesser degree than observed with non PEG IFNs. When fibrosis is assessed as a continuous variable [18], a clear cut inverse relationship with response emerges at all stages. It is thus very likely that, in the presence of more advanced liver disease, a short treatment schedule would not obtain adequate rates of SVR. Since viraemia tends to be lower when cirrhosis is more advanced, this issue may have practical relevance.
Notwithstanding all the methodological criticisms, Zeuzem and co-workers must be commended for pushing the issue of optimization beyond the conventional wisdom of genotype, with a large single-arm trial for also taking into consideration both quality and early assessment of viral drop. There is an evident need for studies with truly prospective recruitment and observation and a more articulate panel of options not only in terms of length but also of dosing of PEG IFN and ribavirin. Also, if important treatment decisions are to be made on viral loads, then standardization of HCV-RNA tests and their prompt availability become paramount.
At the present time, the strength of the evidence presented by Zeuzem and co-workers is in our opinion not sufficient to generalize the applicability of this approach to optimization. While further randomized trials are in progress, subjects with genotype 1, a low viral load and an early drop of HCV-RNA under therapy may be treated with this short schedule if they have modest or no fibrosis and hence are unlikely to progress significantly should HCV relapse. Those with more advanced fibrosis are probably still better served by the conventional, more troublesome schedule of 48 weeks.
PUBLISHED STUDY DETAILS CONTINUED
Efficacy of 24 weeks treatment with peginterferon alfa-2b plus ribavirin in patients with chronic hepatitis C infected with genotype 1 and low pretreatment viremia
Introduction
Hepatitis C virus (HCV) infection may progress to chronic hepatitis, cirrhosis, and its sequelae [1-3]. Treatment of HCV-infected patients with interferon-â┐ can achieve viral clearance and improve histology and prognosis [4,5]. In the era of standard interferon alfa plus ribavirin, the duration of treatment in patients with chronic hepatitis C was tailored according to HCV genotype and baseline viremia; patients infected with HCV genotype 1 (HCV-1) and high baseline viremia were treated for 48 weeks, while patients infected with HCV-1 and low baseline viremia as well as all patients infected with HCV-2 or HCV-3 were treated for 24 weeks [6-8].
More recently, standard interferons have been chemically modified using polyethylenglycol (PEG) to improve antiviral efficacy. Higher sustained virologic response rates in patients with chronic hepatitis C have been reported for the pegylated forms of interferons compared with standard interferons both in monotherapy as well as in combination therapy with ribavirin [9-12]. In these trials patients were treated for 48 weeks. Additional prospective trials in patients chronically infected with genotypes HCV-2 or HCV-3 showed that the treatment duration can be reduced from 48 to 24 weeks without compromising antiviral efficacy [13,14]. Data regarding optimal duration of treatment for patients infected with HCV-1, however, are sparse.
The aim of the present study was to investigate efficacy and safety of peginterferon alfa-2b plus ribavirin administered for 24 weeks in patients chronically infected with HCV-1 and a low baseline serum HCV RNA concentration.
Results
For this study, which was performed between October 2001 and October 2004 in 43 European centers, 724 patients were screened and 237 patients were enrolled. Two patients did not have adequate information for analysis, and, therefore, will be presented in demographics and adverse events, but not in the efficacy analyses. Of the remaining 235 patients, two did not receive treatment and are included in the efficacy analysis as nonresponders. The baseline characteristics of the patients are summarized in Table 1.
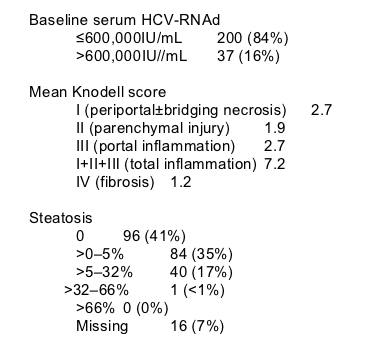
(d)Pretreatment HCV RNA levels were determined at the screening and entry visits. Protocol eligibility was determined from the screening viral load. However, for purposes of analysis, the pretreatment virologic sample closest (in time) to the enrollment date was used to determine the baseline viral load. All 37 patients who had a baseline viral load of >600,000IU/mL had a viral load of ≦600,000IU/mL at the screening determination.
Biochemical and virological response
An overall intent-to-treat virologic response at the end of therapy was achieved by 189 of 235 patients (80%) and a sustained virologic response by 117 of 235 patients (50%). Using the data of the study by Manns et al. [11], a sustained virologic response rate of 69% was predicted if the present study cohort was treated for 48 weeks. This estimated sustained virologic response rate fell outside the 95% confidence interval (43-56%) of the observed virologic response rate after 24 weeks treatment in the study cohort and therefore did not meet the criterion for establishing effectiveness specified for the present study.
At the end of follow-up 134 of 235 patients (57%) had ALT levels within the normal reference range and 110 of 235 patients (47%) had both normal ALT and undetectable HCV-RNA. The correlation between sustained biochemical and virologic response was 94%. ALT levels in sustained virologic but not biochemical responders (n=5) ranged from 1.03 to 1.25 times the upper limit of normal. However, all five patients had lower ALT values at the end of follow-up compared with pretreatment levels. On the pretreatment liver biopsy two of the five patients had grade 2 steatosis (>5-≦32% fat), 2 patients had grade 1 steatosis (>0-≦5% fat), and one patient did not have a biopsy sample submitted.
Predictors of response
Single-variable analysis identified baseline HCV-RNA level, duration of treatment, and age as potential predictors of response. After stepwise multivariable logistic regression analysis, baseline HCV-RNA level (P<0.0001) and treatment duration for at least 16 weeks (P=0.0135) remained significant independent predictors of sustained virologic response (Table 2). Body weight was not a predictor of sustained virologic response by either single-variable or stepwise analysis.
Undetectable serum HCV-RNA after 4 weeks of therapy was an important predictor of sustained virologic response. The sustained virologic response rate was 89% for those who were first HCV-RNA negative at week 4, compared to 25 and 17% in those who had their first undetectable HCV-RNA at week 12 or 24, respectively. Patients who had undetectable HCV-RNA at weeks 4 and 24 of treatment had a sustained response rate of 92%.
The classification tree model demonstrated that a baseline HCV-RNA level of 250,000IU/mL best discriminated between patients with or without sustained virologic response. Patients with a baseline HCV-RNA ≦250,000IU/mL had a significantly higher sustained virologic response rate of 67% (93/138) compared with 25% (24/97) in patients with a baseline HCV-RNA level >250,000IU/mL (P<0.0001). This was due to both a higher end of treatment virologic response rate (89 vs 68%) and a lower confirmed virologic relapse rate (23 vs 63%) in patients with baseline HCV-RNA levels below 250,000IU/mL. Initial viral load and early response were linked. In patients with HCV-RNA concentration of ≦250,000IU/mL, 68% (94/138) had their first undetectable serum HCV-RNA after 4 weeks of treatment compared with 16% (16/97) with baseline HCV-RNA concentration of >250,000IU/mL.
Baseline viral load
Thirty-seven patients (16%) had a screening viral load of ≦600,000IU/mL, followed by a viral load of >600,000IU/mL before initiating treatment. This finding is consistent with the known -0.5log assay variation of real-time PCR testing wherein a proportion of patients with initial viral loads close to the 600,000IU/mL screening cut-off should retest higher than 600,000IU/mL. This finding reinforces the concept that one should not attribute clinical significance to small changes (<1log) in serum viral titers of individual patients with hepatitis C virus infection.
Adherence
Two hundred twenty-one (94%) patients completed at least 80% of the planned treatment duration. Patients were considered adherent to the treatment regimen if they received at least 80% of the assigned dose of both drugs for at least 80% of the treatment duration [16]. Patients who were able to adhere to the assigned treatment regimen were compared with patients who received less than 80% of one or both drugs or completed less than 80% of the treatment duration. The sustained virologic response rate in adherent patients was 52% (102 of 195 patients) compared with 54% (14 of 26 patients) in nonadherent patients. Dose reduction did not seem to affect sustained virologic response rates among HCV-1 infected patients with low baseline HCV-RNA levels, but this observation is based on a small sample size, since only 12% (26/221) were considered nonadherent. Therapy for at least 16 weeks, however, was a strong predictor of sustained virologic response.
Adverse events
Serious adverse events (SAE) were reported in 25 patients during the treatment period, representing an SAE rate of 11%. In 19 of the 25 patients the event was considered by the investigator as probably or possibly related to study medication (depression, asthenia, abdominal pain, fever, anemia, neutropenia, hypocalcemia, pruritus, rash, psoriasis, allergy, thyroiditis, hearing impairment). Seven of 237 patients (3%) discontinued therapy because of adverse events. Two patients discontinued due to depression and one patient each for asthenia, anemia, asthenia plus anemia, breast cancer and psoriasis, respectively.
Sixty-one of 237 patients (26%) required dose reduction or interruption due to adverse events (excluding patients who later discontinued due to an adverse event). Anemia (12%) thrombocytopenia (4%), and neutropenia (3%) were the most common adverse events leading to dose modification. Dose modifications were lower in those patients receiving 800mg/day of ribavirin (9% or 8/87 patients), but similar in patients receiving 1000mg/day and 1200mg/day (14% or 16/114 patients and 15% or 5/34 patients, respectively).
Treatment-emergent SAEs occurred at similar rates in the present 24-week treatment study and the historical control of patients treated for 48 weeks (11 vs 12%) [11]. However, adverse events leading to discontinuation of therapy (3 vs 14%) or dose modifications due to adverse events (26 vs 49%) were considerably lower in the present study compared with the 48-week historical control study [11]. In addition, adverse events leading to discontinuation or dose modifications in this study were lower than those reported in the initial 24 weeks of therapy in the historical control (11 and 41%, respectively) [11].
Discussion
This study demonstrates that 24 weeks of therapy with peginterferon alfa-2b 1.5mg/kg/week plus weight-based ribavirin dosing is insufficient for the treatment of patients infected with HCV-1 and a baseline HCV-RNA level equal or below 600,000IU/mL. Using the data of the study by Manns et al. [11], a sustained virologic response rate of 69% was predicted if the patients in the present study would have been treated for 48 weeks. This estimated sustained virologic response rate fell outside the 95% confidence interval (43-56%) of the observed virologic response rate after 24 weeks treatment in the study cohort and therefore did not satisfy the criterion for establishing effectiveness for the present study.
The classification tree model demonstrated that a pretreatment HCV-RNA level of 250,000IU/mL best discriminated between patients with or without a sustained virologic response after combination therapy with peginterferon alfa-2b and ribavirin for 24 weeks. Future prospective studies should investigate whether only one threshold differentiating between low and high pretreatment viremia is sufficient or whether several ranges of pretreatment HCV-RNA levels could be used to individualize treatment duration. The current definition of low and high viral load originates from the median pretreatment HCV-RNA levels in several pivotal trials, i.e. 2,000,000copies/mL [6,7]. Unfortunately, the conversion according to the WHO standard is currently inconsistent. According to a real-time quantitative PCR assay developed by Schering Plough Research Institute and other assays the threshold between low and high pretreatment viremia is 600,000 or 800,000IU/mL, respectively [11-14]. Furthermore, the natural fluctation of HCV-RNA as well as the intra-assay variability of quantitative assays must be kept in mind.
Data of the present study show that a subgroup of HCV genotype 1 infected patients who had pretreatment HCV RNA levels below 600,000IU/mL and became serum HCV-RNA negative after 4 weeks of treatment achieved an excellent sustained virologic response rate of almost 90% (compared with 85% in the historical control group treated for 48 weeks [11]). These data are in line with a recent prospective study in which treatment of patients with chronic hepatitis C was individualized according to the early virologic response [17]. In that study, serum HCV-RNA was frequently quantified during the initial 6 weeks of peginterferon alfa-2a plus ribavirin combination therapy, and patients were classified as rapid, slow, flat, or null responders. The sustained virologic response rate in HCV-1 infected patients with an initial rapid virologic response who were treated only for 24 weeks was substantial (65%) but was lower than in those patients treated for 48 weeks (83%). This difference was significant in patients with a baseline HCV-RNA >800,000IU/mL, but not in patients with lower baseline viral load [17].
Beyond the initial virologic response at treatment week 4 only two pretreatment parameters (baseline HCV-RNA level and treatment duration for at least 16 weeks) remained significant independent predictors of sustained virologic response in the present study after stepwise multivariable logistic regression analysis. These predictors as well as the fact that body weight was not a predictor of sustained virologic response are in accordance with a similar study in patients infected with HCV genotypes 2 or 3 [14]. However, in the study with HCV-2 or HCV-3 infected patients hepatic steatosis of less than 5% was an additional independent predictor of sustained virologic response [14]. This can be explained because infection particularly with HCV-3 leads to hepatic steatosis [18,19].
Overall, the safety profile of the present 24-week treatment regimen was improved compared with patients in the study of Manns et al. [11] who received more than 10.6mg/kg/day ribavirin for 48 weeks. Adverse events leading to treatment discontinuation or dose reduction occurred at considerably lower rates in the present study compared with the historical control study. Improved adverse event rates in this study were also seen compared with the initial 24 weeks of treatment in the historical control. Over the recent years, investigators may have become more familiar with the medications and the management of their adverse events, leading to this improved safety profile.
In general, patients chronically infected with HCV-1 should receive combination therapy with a pegylated interferon plus weight-based ribavirin for 48 weeks. An exception comprises HCV-1 infected patients with a low pretreatment HCV-RNA concentration (below 600,000IU/mL) who become undetectable for serum HCV-RNA already after 4 weeks of combination therapy. In this subset of patients treatment for 24 weeks does not impair the sustained virologic response rate. Although a baseline HCV-RNA concentration of less than 250,000IU/mL may predict the need for shorter course therapy, the strongest predictor is virologic response after 4 weeks of therapy. Given the intra-assay variability of PCR testing (-0.5log), response after 4 weeks of therapy is the most useful predictor of sustained virologic response in clinical practice.
Patients and methods
Patients
Male and female patients aged 18-70 years with compensated chronic HCV-1 infection not previously treated with interferon, ribavirin and/or amantadine were eligible for enrollment. Eligible patients tested positive for HCV-RNA by reverse transcription-polymerase chain reaction with a concentration ≦600,000IU/mL, had a liver biopsy taken within 12 months prior to the screening visit showing chronic hepatitis, and had at least one elevated serum alanine aminotransferase (ALT) level at screening or entry into the trial. Entry leucocyte and platelet counts had to be at least 3000 and 80,000/mL, respectively. Hemoglobin values at entry visit had to be at least 12g/dL for females and at least 13g/dL for males.
Patients with the following criteria were excluded: any other cause of liver disease or other relevant disorders including human immunodeficiency or hepatitis B virus coinfection, clinically significant hematologic, hepatic, metabolic, renal, rheumatologic, anaphylactic reactions, neurological or psychiatric disease, clinically significant cardiac or cardiovascular abnormalities, organ grafts, systemic infection, clinically significant bleeding disorders, evidence of malignant neoplastic disease, average daily intake of alcohol exceeding 80g of ethanol, or drug abuse within the past two years. Further exclusion criteria were pregnancy and lactation.
Study design
This phase 4, single-arm, open-label, historical-control study assessed the safety and efficacy of 24 weeks of treatment with peginterferon alfa-2b plus ribavirin in previously untreated patients with chronic hepatitis C who were infected with HCV-1 with a screening HCV-RNA concentration ≦600,000IU/mL. Eligible patients were treated with peginterferon alfa-2b (PegIntron , Schering Plough Corp., Kenilworth, NJ) 1.5mg/kg once per week subcutaneously plus ribavirin (Rebetol , Schering Plough Corp., Kenilworth, NJ) 800-1400mg/day orally (<65kg, 800mg; 65-85kg, 1000mg; >85-105kg, 1200mg; >105kg, 1400mg). Weight-based dosing of ribavirin (>10.6mg/kg/day) was selected to provide the standard of care dosing regimen as well as the optimal dosing regimen with regard to safety and efficacy as established in the historical control [11].
The dose of each study medication was based on the patient's weight at entry. Patients were treated for 24 weeks, then followed for an additional 24 weeks. The study was approved by the ethics committees at the participating centers and conducted according to the Declaration of Helsinki and the ICH/CPMP guidelines 'Good Clinical Practice'. All patients gave written informed consent before enrollment.
Patients were evaluated as outpatients for safety, tolerance, and efficacy at weeks 4, 8, 12, 18, and 24 during treatment and at weeks 4, 12, and 24 following the end of treatment. HCV-RNA was quantified by real-time polymerase chain reaction technology (lower limit of detection 29IU/mL). HCV genotyping was performed by direct sequencing of the 5'-noncoding region. Liver biopsy specimens were assessed by an experienced pathologist who was unaware of clinical and biochemical data as well as of treatment regimen and response. Steatosis was graded according to the percentage of hepatocytes containing visible macrovesicular steatosis. Histological results were classified according to internationally standardized criteria [15].
Study end points
The primary efficacy endpoint for this study was the proportion of patients with a sustained virologic response, defined as undetectable plasma HCV-RNA levels at 24 weeks following the end of treatment. The secondary endpoint was the proportion of patients with a sustained virologic response and normalization of alanine aminotransferase (ALT) at the end of the follow-up period. The safety and efficacy of 24 weeks of treatment in this trial were compared with the historical control that treated patients with combination therapy including >10.6mg/kg/day of ribavirin for 48 weeks [11]. Safety data were also compared with the initial 24 weeks of therapy in the historical control.
Statistical analysis
The primary efficacy analysis was based on 235 enrolled patients. A prediction model for sustained virologic response was developed using data from Manns et al. [11]. This model included the following prognostic factors: genotype, baseline HCV-RNA level, presence or absence of bridging fibrosis (F3) or cirrhosis (F4), age and gender. The model was then used to predict sustained virologic response rates for the HCV-1 infected patients with low baseline viremia in the present study, had they received 48 weeks of treatment. If the estimated sustained virologic response rate for 48 weeks of treatment based on the model fell within the 95% confidence interval of the actually observed sustained virologic response rate of the present study, then it was concluded that 24 weeks of treatment is equivalent to 48 weeks of treatment.
Single-variable logistic regression was used to compute p-values and odds ratios for the effect of prognostic factors (those observed at baseline and also treatment duration) upon sustained response. Stepwise regression analysis was performed to determine the significance of the results from the single-variable logistic regression analysis. The classification tree approach (SAS Enterprise Miner, Tree model using the Chi-square splitting criterion) determined the relationship between baseline viral load and sustained virologic response.
|
|
| |
| |
|
|
|