| |
CDC Recommends Post HIV Exposure Prophylaxis For All
|
| |
| |
The Federal government for the first time is recommending that people who are exposed to HIV/AIDS receive drug cocktails (HIV therapy) in the hopes that this will prevent HIV infection. Below you will find the CDC Press Release. An Associated Press article, and the Guidelines themselves from the CDC website.
EXCERPTS FROM GUIDELINES
|
|
| |
| |
 |
|
| |
| |
".....Evidence of Possible Benefits from nPEP.....
For ethical and logistical reasons, a randomized, placebo-controlled clinical trial of nPEP probably will not be performed. However, data are available from animal transmission models, perinatal clinical trials, studies of health-care workers receiving prophylaxis after occupational exposures, and from observational studies. These data indicate that nPEP might sometimes reduce the risk for HIV infection after nonoccupational exposures......
"........animal studies have demonstrated mixed results. In macaques, PMPA (tenofovir) blocked simian immunodeficiency virus (SIV) infection after intravenous challenge if administered within 24 hours of exposure and continued for 28 days. PMPA was not as effective if initiated 48 or 72 hours postexposure or if continued for only 3 or 10 days. Two macaque studies of combination antiretroviral therapy (zidovudine, lamivudine, and indinavir) initiated 4 hours after simian/human immunodeficiency virus (SHIV) challenge and continued for 28 days did not protect against infection but did result in reduced viral load among the animals infected. In a macaque study designed to model nPEP for mucosal HIV exposure, all animals administered PMPA for 28 days, beginning 12 hours (four animals) or 36 hours (four animals) after vaginal HIV-2 exposure, were protected. Three of four animals treated 72 hours after exposure were also protected; the fourth animal had delayed seroconversion and maintained a low viral load after treatment......
".......Men who began taking nPEP after a self-identified high-risk exposure were evaluated within 96 hours; 92% met the event eligibility criteria (clinician-defined high-risk exposure). Seroincidence was 0.7 per 100 person-years (one seroconversion) among men who took nPEP and 4.1 per 100 person-years among men who did not take nPEP (11 seroconversions).....".
|
|
| |
| |
 |
|
| |
| |
|
|
| |
| |
 |
|
| |
| |
CDC Recommends HIV Drugs for All Exposed:
By DANIEL YEE
The Associated Press
ATLANTA (AP) - In a major policy shift, the government recommended for the first time Thursday that people exposed to the AIDS virus from rapes, accidents or occasional drug use or unsafe sex receive drug cocktails that can keep them from becoming infected.
Previously, federal health officials recommended emergency drug treatment only for health-care workers accidentally stuck with a needle, splashed in the eye with blood, or exposed in some other way on the job. That recommendation was first made in 1996.
The Centers for Disease Control and Prevention expanded its guidelines to rape victims and many others Thursday. It said treatment should start no more than 72 hours after a person has been exposed to the virus, and the drugs should be used by patients for 28 days.
It is a major shift away from a policy that some doctors had called unconscionable and that put the United States years behind much of Europe and other nations.
``The severity of the HIV epidemic dictates we use all available tools to reduce infection,'' said Dr. Ronald Valdiserri of the CDC.
He stressed that emergency drug treatment is a ``safety net,'' not a substitute for abstinence, monogamy, and the use of condoms and sterile needles.
``It is clearly not a `morning-after pill,''' he said.
People accidentally exposed to the AIDS virus are usually given a three-drug combination that includes AZT and 3TC.
In tests on primates, drug cocktails prevented infection with the monkey version of HIV 100 percent of the time if given within 24 hours of exposure to the virus, and 52 percent of the time if administered within 72 hours, said Dr. Charles Gonzalez, assistant professor of medicine at New York University School of Medicine and a member of the New York State AIDS Institute medical guidelines board.
However, there is no data from clinical trials on how effective the drugs are in stemming HIV infection in people.
The new guidelines do not bind the U.S. government to pay for the treatment regimen through Medicare or Medicaid, and no federal money has been allocated to help doctors and health departments carry out the recommendations.
European countries, Australia and Brazil have long had guidelines calling for the use of HIV drugs to prevent infection in rape victims. Without a national policy in the United States, New York, California, Massachusetts and Rhode Island and cities such as San Francisco and Boston came up with their own such guidelines.
``It's unconscionable they didn't have a policy for rape victims. It's just ludicrous. They knew they were well behind the curve,'' Gonzalez said.
The CDC said it hesitated to recommend wider use of AIDS drugs because it did not have enough information on their effectiveness. But the agency said better information has been gathered over the past several years from animal and lab studies and from state and city programs that offer HIV drugs to rape victims and others.
But Gonzalez suggested that the CDC may have been stymied by a conservative administration in Washington from making such a recommendation from earlier.
``This may be a 'red state, blue state' issue, where states such as Massachusetts, New York and California are willing to discuss this matter in terms of rape and what happens to consensual adults,'' Gonzalez said.
The CDC said the regimen is not recommended for habitual drug users who share needles or for people who frequently engage in risky sex. Those people would have to take medication practically nonstop, which the health agency does not endorse.
On the Net:
CDC info: http://www.cdc.gov
CDC Press Release - HIV Post-Exposure Prophylaxis Guidelines
Dear Colleague:
New HIV prevention interventions are urgently needed for gay and bisexual men in the U.S. Data show that HIV/AIDS diagnoses among men who have sex with men (MSM) in 32 states are on the rise. And while most gay men take steps to reduce their HIV risk, condom breakage or occasional lapses in safer behavior can still place them at risk for infection.
To provide an HIV prevention safety net, the U.S. government today issued national guidelines on the use of post-exposure antiretroviral treatment for people exposed to the virus through sexual intercourse, sexual assault, injection drug use or accidents. This approach, called non-occupational post-exposure prophylaxis (NPEP), involves taking a daily antiretroviral regimen within 72 hours of exposure and continuing it for 28 days to stop HIV infection from taking hold in the body.
NPEP is an important expansion of current HIV prevention strategies and, and if used appropriately with other proven prevention methods, could help reduce new infections. However, NPEP should not be viewed as the first-line defense against HIV, and does not replace abstinence, mutual monogamy, consistent condom use, safer injection drug-use practices and other behaviors that can help people avoid being exposed to HIV in first place.
The press release announcing the NPEP guidelines and a media backgrounder are attached. The guidelines themselves, dated January 21, 2005, are available online at www.cdc.gov/mmwr/mmwr_rr.html.
Please do not hesitate to contact me for more information or to arrange an interview with a CDC spokesperson.
Best regards,
Sara Satinsky
T. 212-584-5007
Antiretroviral Postexposure Prophylaxis After Sexual, Injection-Drug Use, or Other Nonoccupational Exposure to HIV in the United States
Recommendations from the U.S. Department of Health and Human Services
1Division of HIV/AIDS Prevention, National Center for HIV, STD, and TB Prevention, CDC, Atlanta, Georgia
2National Institutes of Health
3Food and Drug Administration, Washington, D.C.
4Health Resources and Services Administration
The material presented in this report originated in the Division of HIV/AIDS Prevention, National Center for HIV, STD, and TB Prevention, Janet L. Collins, MD, Acting Director.
Corresponding Author: Lisa A. Grohskopf, MD, Epidemiology Branch, Division of HIV/AIDS Prevention, National Center for HIV, STD and TB Prevention, CDC, 1600 Clifton Road NE, MS-E45, Atlanta, GA 30333. Telephone: 404-639-6116; Fax: 404-639-6127; e-mail: lkg6@cdc.gov.
Summary
The most effective means of preventing human immunodeficiency virus (HIV) infection is preventing exposure. The provision of antiretroviral drugs to prevent HIV infection after unanticipated sexual or injection-drug--use exposure might be beneficial. The U.S. Department of Health and Human Services (DHHS) Working Group on Nonoccupational Postexposure Prophylaxis (nPEP) made the following recommendations for the United States. For persons seeking care <72 hours after nonoccupational exposure to blood, genital secretions, or other potentially infectious body fluids of a person known to be HIV infected, when that exposure represents a substantial risk for transmission, a 28-day course of highly active antiretroviral therapy (HAART) is recommended. Antiretroviral medications should be initiated as soon as possible after exposure. For persons seeking care <72 hours after nonoccupational exposure to blood, genital secretions, or other potentially infectious body fluids of a person of unknown HIV status, when such exposure would represent a substantial risk for transmission if the source were HIV infected, no recommendations are made for the use of nPEP. Clinicians should evaluate risks and benefits of nPEP on a case-by-case basis. For persons with exposure histories that represent no substantial risk for HIV transmission or who seek care >72 hours after exposure, DHHS does not recommend the use of nPEP. Clinicians might consider prescribing nPEP for exposures conferring a serious risk for transmission, even if the person seeks care >72 hours after exposure if, in their judgment, the diminished potential benefit of nPEP outweighs the risks for transmission and adverse events. For all exposures, other health risks resulting from the exposure should be considered and prophylaxis administered when indicated. Risk-reduction counseling and indicated intervention services should be provided to reduce the risk for recurrent exposures.
Introduction
The most effective methods for preventing human immunodeficiency virus (HIV) infection are those that protect against exposure to HIV. Antiretroviral therapy cannot replace behaviors that help avoid HIV exposure (e.g., sexual abstinence, sex only in a mutually monogamous relationship with a noninfected partner, consistent and correct condom use, abstinence from injection-drug use, and consistent use of sterile equipment by those unable to cease injection-drug use). Medical treatment after sexual, injection-drug--use, or other nonoccupational HIV exposure* is less effective than preventing HIV infection by avoiding exposure.
In July 1997, CDC sponsored the External Consultants Meeting on Antiretroviral Therapy for Potential Nonoccupational Exposures to HIV. This panel of scientists, public health specialists, clinicians, ethicists, members of affected communities, and representatives from professional associations and industry evaluated the available evidence related to use of antiretroviral medications after nonoccupational HIV exposure. In 1998, DHHS issued a statement that outlined the available information and concluded that evidence was insufficient about the efficacy of nonoccupational postexposure prophylaxis (nPEP) to recommend either for or against its use (1).
Since 1998, additional data about the potential efficacy of nPEP have accumulated from human, animal, and laboratory studies. Clinicians and organizations have begun providing nPEP to patients they believe might benefit. In certain instances, health departments have issued advisories or recommendations or otherwise supported the establishment of nPEP treatment programs in their jurisdictions (2--6). In May 2001, CDC convened the second external consultants meeting on nonoccupational post-exposure prophylaxis to review and discuss the available data. This report summarizes knowledge about the use and potential efficacy of nPEP and details guidelines for its use in the United States.† The recommendations are intended for nonoccupational exposures and are not applicable for occupational exposures.
Evidence of Possible Benefits from nPEP
For ethical and logistical reasons, a randomized, placebo-controlled clinical trial of nPEP probably will not be performed. However, data are available from animal transmission models, perinatal clinical trials, studies of health-care workers receiving prophylaxis after occupational exposures, and from observational studies. These data indicate that nPEP might sometimes reduce the risk for HIV infection after nonoccupational exposures.
Animal Studies
Animal studies have demonstrated mixed results (1,7). In macaques, PMPA (tenofovir) blocked simian immunodeficiency virus (SIV) infection after intravenous challenge if administered within 24 hours of exposure and continued for 28 days. PMPA was not as effective if initiated 48 or 72 hours postexposure or if continued for only 3 or 10 days (8). Two macaque studies of combination antiretroviral therapy (zidovudine, lamivudine, and indinavir) initiated 4 hours after simian/human immunodeficiency virus (SHIV) challenge and continued for 28 days did not protect against infection but did result in reduced viral load among the animals infected (9). In a macaque study designed to model nPEP for mucosal HIV exposure, all animals administered PMPA for 28 days, beginning 12 hours (four animals) or 36 hours (four animals) after vaginal HIV-2 exposure, were protected. Three of four animals treated 72 hours after exposure were also protected; the fourth animal had delayed seroconversion and maintained a low viral load after treatment (10).
These findings are consistent with those of macaque studies of the biology of vaginal SIV transmission. After atraumatic vaginal inoculation, lamina propria cells of the cervicovaginal subepithelium were infected first, virus was present in draining lymph nodes within 2 days, and virus was disseminated to the blood stream by 5 days (11). Similarly, in another study, SIV-RNA was detected in dendritic cells from the vaginal epithelium within 1 hour of intravaginal viral exposure, and SIV-infected cells were detected in the lymph nodes within 18 hours (12). These data indicate a small window of opportunity during which it might be possible to interrupt either the initial infection of cells in the cervicovaginal mucosa or the dissemination of local infection by the prompt administration of antiretroviral medications.
Postnatal Prophylaxis
Abbreviated regimens for reducing mother-to-child HIV transmission have been studied extensively. Certain regimens have included a postexposure component (antiretroviral medications given to the neonate). Although reduction in maternal viral load during late pregnancy, labor, and delivery seems to be a major factor in the effectiveness of these regimens, an additional effect is believed to occur because the neonate receives prophylaxis, which protects against infection from exposure to maternal HIV during labor and delivery (13,14). In a Ugandan perinatal trial, the rate of transmission at 14--16 weeks postpartum was substantially lower for women who received a single dose of nevirapine at the beginning of labor followed by a single dose of nevirapine to the neonate within 72 hours of birth (transmission rate: 13.1%) than for the women who received intrapartum zidovudine followed by 1 week of zidovudine to the neonate (transmission rate: 25.1%) (15). Similarly low transmission rates were noted in a study in South Africa in which intrapartum and postpartum antiretroviral medications were used. At 8 weeks postpartum, the transmission rate was 9.3% after intrapartum zidovudine and lamivudine followed by 1 week of zidovudine and lamivudine to mother and neonate, and the transmission rate was 12.3% after a single dose of nevirapine administered to the mother during labor and then to the neonate within 72 hours of birth (16). Although these studies lacked control groups, these dosing schedules could not have substantially reduced HIV exposure of the neonate through reducing maternal viral load, demonstrating that a combination of pre-exposure and postexposure prophylaxis for the neonate reduces HIV transmission. A study in Malawi among women who did not receive intrapartum antiretrovirals compared postnatal prophylaxis with single-dose nevirapine with and without zidovudine for 1 week. The transmission rate at 6--8 weeks was 7.7% among infants who received zidovudine plus nevirapine compared with 12.1% among those who received nevirapine alone (17). Although this study did not have a placebo or no-prophylaxis arm, the transmission rate for the zidovudine-nevirapine arm compares favorably with the rate of 21% at 4 weeks, noted in the placebo arm of a study of zidovudine prophylaxis conducted in Cote d'Ivoire (18).
Two observational studies with relatively limited numbers documented a potential effect of postnatal zidovudine prophylaxis alone (without intrapartum medication). A review of medical records in New York indicated that zidovudine monotherapy administered to the mother intrapartum or to the infant within 72 hours of birth reduced perinatal transmission >50%; initiating monotherapy for the infant >72 hours after birth was less effective (19). Similarly, an analysis of births in the PACTS study demonstrated that zidovudine administered to infants within 24 hours of birth, when mothers had not been treated either antepartum or intrapartum, compared with no treatment for mothers or infants, reduced perinatal transmission by 48% (20).
Observational Studies of nPEP
The most direct evidence supporting the efficacy of postexposure prophylaxis is a case-control study of needlestick injuries to health-care workers. In this study, the prompt initiation of zidovudine was associated with an 81% decrease in the risk for acquiring HIV (21). Although analogous clinical studies of nPEP have not been conducted, data are available from observational studies and registries.
In a high-risk HIV incidence cohort in Brazil, nPEP instruction and 4-day starter packs of zidovudine and lamivudine were administered to 200 homosexual and bisexual men. Men who began taking nPEP after a self-identified high-risk exposure were evaluated within 96 hours; 92% met the event eligibility criteria (clinician-defined high-risk exposure). Seroincidence was 0.7 per 100 person-years (one seroconversion) among men who took nPEP and 4.1 per 100 person-years among men who did not take nPEP (11 seroconversions) (22,23). Subsequent analysis of data from patients who took nPEP and had been followed for a median of 24.2 months indicated 11 seroconversions and a seroincidence of 2.9 per 100 person-years, compared with an expected seroincidence of 3.1 per 100 person years, p>0.97) (24). In a study of sexual assault survivors in Sao Paolo, Brazil, women who sought care within 72 hours after exposure were treated for 28 days with either zidovudine and lamivudine (for those without mucosal trauma) or zidovudine, lamivudine, and indinavir (for those with mucosal trauma or those subjected to unprotected anal sex) for 28 days. Women were not treated if they sought care >72 hours after assault, if the assailant was HIV-negative, or if a condom was used and no mucosal trauma was seen. Of 180 women treated, none seroconverted. Of 145 women not treated, four (2.7%) seroconverted (25). Although these studies demonstrate that nPEP might reduce the risk for infection after sexual HIV exposures, participants were not randomly assigned, and sample sizes were too small for statistically significant conclusions.
In a study of rape survivors in South Africa, of 480 initially seronegative survivors begun on zidovudine and lamivudine and followed up for at least 6 weeks, one woman seroconverted. She had started taking medications 96 hours after the assault. An additional woman, who sought treatment 12 days after assault, was seronegative at that time but not offered nPEP. At retesting 6 weeks after the assault, she had seroconverted and had a positive polymerase chain reaction result (Personal communication, A. Wulfsohn, MD, Sunninghill Hospital, Gauteng, South Africa).
In a feasibility trial of nPEP conducted in San Francisco, 401 persons with eligible sexual and injection-drug--use exposures were enrolled. No seroconversions were observed among those who completed treatment, those who interrupted treatment, or those who did not receive nPEP (26). In a study in British Columbia of 590 persons who completed a course of nPEP, no seroconversions were observed (27). In registries from four countries (Australia, France, Switzerland, and the United States), including approximately 2,000 nonoccupational exposure case reports, no confirmed seroconversions have been attributed to a failure of nPEP in approximately 350 nPEP-treated persons reported to have been exposed to HIV-infected sources. However, the absence of seroconversions might not be attributed to receipt of nPEP but rather to the low per-act risk for infection and incomplete follow-up in the registries.
Case Reports
In addition to these studies, two case reports are of note. In one, a patient who received a transfusion of red blood cells from a person subsequently determined to have early HIV infection began taking combination PEP 1 week after transfusion and continued for 9 months. The patient did not become infected despite the high risk associated with the transfusion of HIV-infected blood (28). In the other case, nPEP was initiated 10 days after self-insemination with semen from a homosexual man later determined to have early HIV infection. The woman did not become infected but did become pregnant and gave birth to a healthy infant (29).
Although data from the studies and case reports do not provide definitive evidence of the efficacy of nPEP after sexual, injection-drug--use, and other nonoccupational exposures to HIV, the cumulative data demonstrate that antiretroviral therapy initiated soon after exposure and continued for 28 days might reduce the risk for acquiring HIV.
Evidence of Possible Risks from nPEP
Concerns about the potential risks from nPEP as a public health intervention include possible decrease in risk-reduction behaviors resulting from a perception that postexposure treatment is available, the occurrence of serious adverse effects from antiretroviral treatment in otherwise healthy persons, and potential selection for resistant virus (particularly if adherence is poor during the nPEP course). Evidence indicates that these theoretical risks might not be major problems.
Effects on Risk-Reduction Behaviors
The availability or use of nPEP might not lead to increases in risk behavior. Of participants in the nPEP feasibility study in San Francisco, 72% reported a decrease in risk behavior over the next 12 months relative to baseline reported risk behavior, 14% reported no change, and 14% reported an increase (30). However, 17% of participants requested a second course of nPEP during the year after the first course, indicating that although participants did not increase risk behaviors, a substantial proportion of the participants did not eliminate risk behaviors. A similar proportion of participants (14%) requested a second course of nPEP at the Fenway Clinic in Boston (31). In the Brazil nPEP study of homosexual and bisexual men followed up for a median of 24 months, all groups, including those who elected to take nPEP, reported decreases in risk behavior (24,32). Among highly educated (75% with >4 years of college), predominantly white (74%) homosexual men who completed a street-outreach interviewer-administered survey in San Francisco, those who reported that they were aware of the availability of nPEP did not report more risk behavior than those who were not aware (33). In a study of discordant heterosexual couples, none reported decreased condom use because of the availability of nPEP (34).
Antiretroviral Side Effects and Toxicity
Initial concerns about severe side effects and toxicities have been ameliorated by experience with health-care workers who have taken PEP after occupational exposures. Of 492 health-care workers reported to the occupational PEP registry, 63% took at least three medications. Overall, 76% of workers who received PEP and had 6 weeks of follow-up reported certain symptoms (i.e., nausea [57%] and fatigue or malaise [38%]). Only 8% of these workers had laboratory abnormalities, few of which were serious and all of which resolved promptly at the end of antiretroviral treatment (35). Six (1.3%) reported severe adverse events, and four stopped taking PEP because of them. Of 68 workers who stopped taking PEP despite exposure to a source person known to be HIV-positive, 29 (43%) stopped because of side effects. According to the U.S. nPEP surveillance registry, among 107 exposures for which nPEP was taken, the regimen initially prescribed was stopped or modified in 22%; modifications or stops were reported because of side effects in half of these instances (36). In addition to reports in these registries, serious side effects have been reported (e.g., nephrolithiasis and hepatitis) in the literature.
During 1997--2000, a total of 22 severe adverse events in persons who had taken nevirapine-containing regimens for occupational or nonoccupational postexposure prophylaxis were reported to FDA (37--38). Severe hepatotoxicity occurred in 12 (one requiring liver transplantation), severe skin reactions in 14, and both hepatic and cutaneous manifestations occurred in four. Because the majority of occupational exposures do not lead to HIV infection, the risk for using a nevirapine-containing regimen for occupational PEP outweighs the potential benefits. The same rationale indicates that nevirapine should not be used for nPEP.
Selection of Resistant Virus
Antiretroviral PEP does not prevent all infections in occupational and perinatal settings. Similarly, PEP is not expected to have complete efficacy after nonoccupational exposures. In instances where nPEP fails to prevent infection, selection of resistant virus by the antiretroviral drugs is theoretically possible. However, because of the relative paucity of documented nPEP failures for which resistance testing was performed, the likelihood of this occurring is unknown.
PEP failures have been documented after at least one sexual (39) and 21 occupational (38,40) exposures. Three fourths of these patients were treated with zidovudine monotherapy. Only three received three or more antiretroviral medications for PEP. Among the patients tested, several were infected with strains that were resistant to antiretroviral medications. In a study in Brazil (24), virus obtained on day 28 of therapy from the only treated person who seroconverted (whose regimen included 3TC) had a 3TC-resistance mutation. However, the source-person could not be tested. Therefore, whether the mutation was present when the virus was transmitted or whether it developed during nPEP could not be determined.
Selection of resistant virus might occasionally result from the use of nPEP. However, because the majority of nonoccupational exposures do not lead to HIV infection and because the use of combination antiretroviral therapy might reduce further the transmission rate, such occurrences are probably rare. For patients who seroconvert despite nPEP, resistance testing should be considered to guide early and subsequent treatment decisions.
Cost-Effectiveness of nPEP
Although the potential benefits of nPEP to persons are measured by balancing its anticipated efficacy after a given exposure against individual health risks, the value of nPEP as a public health intervention is best addressed at the population level by using techniques such as cost-benefit analysis. Such analyses have been published. One cost-effectiveness evaluation of nPEP in different potential exposure scenarios in the United States reported it to be cost-effective only in situations in which the sex partner source was known to be HIV-infected or after unprotected receptive anal intercourse with a homosexual or bisexual man of unknown serostatus (41,42). A similar analysis in France reported that nPEP was cost-saving for unprotected receptive anal intercourse with a partner known to be HIV-infected and cost-effective for receptive anal intercourse with a homosexual or bisexual partner of unknown serostatus. It was not cost-effective for penile-vaginal sex, insertive anal intercourse, or other exposures considered (43).
Another study and anecdotal reports indicate difficulty limiting nPEP to the exposures most likely to benefit from it. In British Columbia, where guidelines for nPEP use have been implemented (5), an analysis indicated that >50% of those receiving nPEP should not, according to the guidelines, have been treated (e.g., for exposure to intact skin). The use of nPEP in these circumstances doubled the estimated cost per HIV infection prevented ($530,000 versus $230,000) (44).
Even if nPEP is cost-effective for the highest risk exposures, behavioral interventions are more cost-effective (41,45). This emphasizes the importance, when considering nPEP, of providing risk-avoidance and risk-reduction counseling to reduce the occurrence of future HIV exposures.
Evidence of Current Practice
Although 40,000 new HIV infections occur in the United States each year, relatively few exposed persons seek care after nonoccupational exposure. Certain exposures are unrecognized. Certain patients have frequently recurring exposures and would not benefit from nPEP because 4 weeks of potential protection cannot substantially reduce their overall risk for acquiring HIV infection. In addition, certain clinicians and exposed patients are unaware of the availability of nPEP or unconvinced of its efficacy and safety. Finally, access to knowledgeable clinicians or a means of paying for nPEP might constrain its use.
Certain populations in the United States remain at high risk for exposure. In a cohort study of homosexual and bisexual men, 17% reported at least one condom failure during the 6 months preceding study enrollment (46). Other studies indicate that increasing use of highly active antiretroviral therapy (HAART) by HIV-infected persons might be leading some persons to have unprotected sex more frequently, in part because of the belief that lowered viral load substantially reduces infectivity (47--50). This finding is supported by increased rates of sexually transmitted infections among HIV-infected patients (51). In a California study, 69% of discordant heterosexual couples reported having had unprotected sex during the preceding 6 months (34).
Since 1998, certain clinicians have recommended wider availability and use of nPEP (52--58), and others have been more cautious about implementing it in the absence of definitive evidence of efficacy (59,60). Multiple public health jurisdictions, including the New York State AIDS Institute, the San Francisco County Health Department, the Massachusetts Department of Public Health, the Rhode Island Department of Health, and the California State Office of AIDS, have issued policies or advisories for nPEP use. Some of these recommendations have focused on sexual assault survivors, who constitute few of the estimated 40,000 new HIV infections annually in the United States.
Surveys of clinicians and facilities indicate a need for more widespread implementation of guidelines and protocols for nPEP use (61). In a survey of Massachusetts emergency department directors, 52% of facilities had received nPEP requests during the preceding year, but only 15% had written nPEP protocols (62). Similarly, in a survey of Massachusetts clinicians, approximately 20% had a written nPEP protocol (63). Among pediatric emergency medicine specialists surveyed throughout the United States and Canada, approximately 20% had a written policy about nPEP use, but 33% had prescribed it for children and adolescents; different prescribing practices were reported (64). In a survey of 27 European Union countries, 23 had guidelines for occupational PEP use, but only six had guidelines for nPEP use (65).
Evidence indicates considerable awareness of nPEP and interest in its use among potential patients. In a cohort study of homosexual and bisexual men, 60% were willing to participate in a study of nPEP if it involved a single daily dose of medication; 30% were willing to take 3 doses daily (66). Among men surveyed at a "gay pride" festival in Atlanta, although only 3% had used nPEP, 26% planned to if exposed in the future (67). When nPEP studies were established in San Francisco, approximately 400 persons sought treatment in 2½ years (24). At a clinic primarily serving homosexual and bisexual men in Boston, 71 requests for nPEP were evaluated in 1½ years (30). In a California study of heterosexual discordant couples, 28% had heard of nPEP, 55% of seronegative partners believed that it was effective, and 78% reported they would take it if exposed (34).
No nationally representative data exists on nPEP use in the United States. In 1998, CDC established a national nPEP surveillance registry that accepts voluntary reports by clinicians. Although approximately 800 reports have been received, the majority of clinicians prescribing nPEP do not report to the registry. Similarly, low reporting rates were obtained in attempts to establish voluntary registries to monitor occupational PEP and antiretroviral use during pregnancy. No national surveys of clinicians have been reported. However, one multisite HIV vaccine trial largely conducted in the United States has assessed nPEP use by 5,418 participants, who included men who have sex with men (94%) and heterosexual women at high risk (6%). Two percent of trial participants from 27 study sites reported having taken nPEP during the trial. Supplementary data from six U.S. sites indicated that 46% of participants had heard of nPEP. Enrollment at one of seven California sites (odds ratio [OR] = 3.2), having a known positive partner (OR = 2.0), higher educational level (OR = 1.4), and greater recreational drug use (OR = 1.2) were significant predictors of having used nPEP (p<0.05) (68).
Evaluation of Persons Seeking Care After Potential Nonoccupational Exposure to HIV
The effective delivery of nPEP after exposures that have a substantial risk for HIV infection requires prompt evaluation of patients and consideration of biomedical and behavioral interventions to address current and ongoing health risks. This evaluation should include determination of the HIV status of the potentially exposed person, the timing and characteristics of the most recent exposure, the frequency of exposures to HIV, the HIV status of the source, and the likelihood of concomitant infection with other pathogens or negative health consequences of the exposure event.
HIV Status of the Potentially Exposed Person
Because persons who are infected with HIV might not be aware they are infected, baseline HIV testing should be performed on all persons seeking evaluation for potential nonoccupational HIV exposure. If possible, this should be done with an FDA-approved rapid test kit (with results available within an hour). If rapid tests are not available, an initial treatment decision should be made based on the assumption that the potentially exposed patient is not infected, pending HIV test results.
Timing and Frequency of Exposure
Available data indicate that nPEP is less likely to be effective if initiated >72 hours after HIV exposure. If initiation of nPEP is delayed, the likelihood of benefit might not outweigh the risks inherent in taking antiretroviral medications.
Because nPEP is not 100% effective in preventing transmission and because antiretroviral medications carry a certain risk for adverse effects and serious toxicities, nPEP should be used only for infrequent exposures. Persons who engage in behaviors that result in frequent, recurrent exposures that would require sequential or near-continuous courses of antiretroviral medications (e.g., discordant sex partners who rarely use condoms or injection-drug users who often share injection equipment) should not take nPEP. In these instances, exposed persons should instead be provided with intensive risk-reduction interventions.
HIV Status of Source
Patients who have had sexual, injection-drug--use, or other nonoccupational exposures to potentially infectious fluids of persons known to be HIV infected are at risk for acquiring HIV infection and should be considered for nPEP if they seek treatment within 72 hours of exposure. If possible, source persons should be interviewed to determine his or her history of antiretroviral use and most recent viral load because this information might provide information for the choice of nPEP medications.
Persons with exposures to potentially infectious fluids of persons of unknown HIV status might or might not be at risk for acquiring HIV infection. When the source is known to be from a group with a high prevalence of HIV infection (e.g., a homosexual or bisexual man, an injection--drug user, or a commercial sex worker), the risk for transmission might be increased. The risk for transmission might be especially great if the source person has been infected recently, when viral burden in blood and semen might be particularly high (69,70). However, ascertaining this in the short time available for nPEP evaluation is rarely possible. When the HIV status of the source is unknown, it should be determined whether the source is available for HIV testing. If the risk associated with the exposure is considered substantial, nPEP can be started pending determination of the HIV status of the source and then stopped if the source is determined to be noninfected.
Transmission Risk from the Exposure
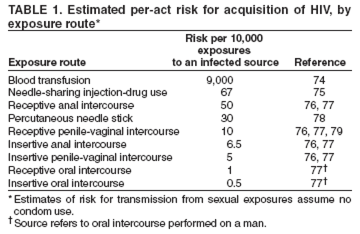
Although the estimated per-act transmission risk from unprotected exposure to a partner known to be HIV infected is relatively low for different types of exposure (Table 1), different nonoccupational exposures are associated with different levels of risk (71--79). The highest levels of estimated per-act risk for HIV transmission are associated with blood transfusion, needle sharing by injection-drug users, receptive anal intercourse, and percutaneous needlestick injuries. Insertive anal intercourse, penile-vaginal exposures, and oral sex represent substantially less per-act risk.
A history should be taken of the specific sexual, injection-drug--use, or other behaviors that might have led to, or modified, a risk for acquiring HIV infection. Eliciting a complete description of the exposure and information about the HIV status of the partner(s) can substantially lower (e.g., if the patient was the insertive partner or a condom was used) or increase (e.g., if the partner is known to be HIV-positive) the estimate of risk for HIV transmission resulting from a specific exposure.
In addition to sexual and injection-drug--use exposures, percutaneous injuries from needles discarded in public settings (e.g., parks and buses) result in requests for nPEP with a certain frequency. Although no HIV infections from such injuries have been documented, concern exists that syringes discarded by injection-drug users (e.g., for whom the HIV infection rate is higher than that for diabetics) might pose a substantial risk. However, these injuries typically involve small-bore needles that contain only limited amounts of blood, and the viability of any virus present is limited. In a study of syringes used to administer medications to HIV-infected persons, only 3.8% had detectable HIV RNA (72). In a study of the viability of virus in needles, viable virus was recovered from 8% at 21 days when the needles had been stored at room temperature; <1% had viable virus after 1 week of storage at higher temperatures (73).
Bite injuries represent another potential means of transmitting HIV. However, HIV transmission by this route has been reported rarely (80-82). Transmission might theoretically occur either though biting or receiving a bite from an HIV-infected person. Biting an HIV-infected person, resulting in a break in the skin, exposes the oral mucous membranes to infected blood; being bitten by an HIV-infected person exposes nonintact skin to saliva. Saliva that is contaminated with infected blood poses a substantial exposure risk. Saliva that is not contaminated with blood contains HIV in much lower titers and constitutes a negligible exposure risk (83).
Evaluation for Sexually Transmitted Infections, Hepatitis, and Emergency Contraception
Evaluation for sexually transmitted infections is important because these infections might increase the risk for acquiring HIV infection from a sexual exposure. In 1996, an estimated 5,042 new HIV infections were attributable to sexually transmitted infection at the time of HIV exposure (84). In addition, any sexual exposure that presents a risk for HIV infection might also place a patient at risk for acquiring other sexually transmitted infections, including hepatitis B. Prophylaxis for sexually transmitted disease, testing for hepatitis, and vaccination for hepatitis B (for those not immune) should be considered (85).
For women of reproductive capacity who have had genital exposure to semen, the risk for pregnancy also exists. In these instances, emergency contraception should be discussed with the potentially exposed patient.
Recommendations for Use of Antiretroviral nPEP
A 28-day course of HAART is recommended for persons who have had nonoccupational exposure to blood, genital secretions, or other potentially infected body fluids of a persons known to be HIV infected when that exposure represents a substantial risk for HIV transmission (Figure 1) and when the person seeks care within 72 hours of exposure. When indicated, antiretroviral nPEP should be initiated promptly for the best chance of success.
Evidence from animal studies and human observational studies demonstrate that nPEP administered within 48--72 hours and continued for 28 days might reduce the risk for acquiring HIV infection after mucosal and other nonoccupational exposures. The sooner nPEP is administered after exposure, the more likely it is to interrupt transmission. Because HIV is an incurable transmissible infection that affects the quality and duration of life, HAART should be used to maximally suppress local viral replication that otherwise might occur in the days after exposure and potentially lead to a disseminated, established infection (11,86). One of the HAART combinations recommended for the treatment of persons with established HIV infection should be selected on the basis of adherence, toxicity, and cost considerations (Tables 2 and 3) (87,88).
No evidence indicates that any specific antiretroviral medication or combination of medications is optimal for use as nPEP. However, on the basis of the degree of experience with individual agents in the treatment of HIV-infected persons, certain agents and combinations are preferred. Preferred regimens include efavirenz and lamivudine or emtricitabine with zidovudine or tenofovir (as a nonnucleoside-based regimen) and lopinavir/ritonavir (coformulated in one tablet as Kaletra®) and zidovudine with either lamivudine or emtricitabine. Different alternative regimens are possible (Table 2).
No evidence indicates that a three-drug HAART regimen is more likely to be effective than a two-drug regimen. The recommendation for a three-drug HAART regimen is based on the assumption that the maximal suppression of viral replication afforded by HAART (the goal in treating HIV-infected persons) will provide the best chance of preventing infection in a person who has been exposed. Clinicians and patients who are concerned about potential adherence and toxicity issues associated with a three-drug HAART regimen might consider the use of a two-drug regimen (i.e., a combination of two reverse transcriptase inhibitors). Regardless of the regimen chosen, the exposed person should be counseled about the potential associated side effects and adverse events that require immediate medical attention. The use of medications to treat symptoms (e.g., antiemetics or antimotility agents) might improve adherence in certain instances.
Although certain preliminary studies have evaluated the penetration of antiretroviral medications into genital tract secretions and tissues (89,90), evidence is insufficient to recommend a specific antiretroviral medication as most effective for nPEP. In addition, new antiretroviral medications might become available. As new medications and new information become available, these recommendations will be amended and updated.
When the source-person is available for interview, his or her history of antiretroviral medication use and most recent viral load measurement should be considered when selecting antiretroviral medications for nPEP. This information might help avoid prescribing antiretroviral medications to which the source-virus is likely to be resistant. If the source-person is willing, the clinician might consider drawing blood for viral load and resistance testing, the results of which might be useful in modifying the initial nPEP medications if the results can be obtained promptly (91).
For persons who have had nonoccupational exposure to potentially infected body fluids of a person of unknown HIV infection status, when that exposure represents a substantial risk for HIV transmission (Figure 1) and when care is sought within 72 hours of exposure, no recommendations are made either for or against the use of antiretroviral nPEP. Clinicians should evaluate the risk for and benefits of this intervention on a case-by-case basis.
When a source-person is not known to be infected with HIV, the risk for exposure (and therefore the potential benefit of nPEP) is unknown. Prescribing antiretroviral medication in these cases might subject patients to risks that are not balanced with the potential benefit of preventing the acquisition of HIV infection. Judging whether the balance is appropriate depends entirely on the circumstances of the possible exposure (i.e., the risk that the source is HIV infected and the risk for transmission if the source is HIV infected) and is best determined through discussion between the clinician and the patient.
If the source-person is available for interview, additional information about risk history can be obtained and permission for an HIV test requested to assist in determining the likelihood of HIV exposure. When available, FDA-approved rapid HIV tests are preferable for obtaining this information as quickly as possible. These additional factors might assist in the decision whether to start or complete a course of nPEP. If data to clearly determine risk are not immediately available, clinicians might consider initiating nPEP while further assessments are being made and then stopping it when other information is available (e.g., the source-person is determined to be noninfected).
For persons whose exposure histories represent no substantial risk for HIV transmission (Figure 1) or who seek care >72 hours after potential nonoccupational HIV exposure, the use of antiretroviral nPEP is not recommended. When the risk for HIV transmission is negligible, limited benefit is anticipated from the use of nPEP. In addition, animal and human study data demonstrate that nPEP is less likely to prevent HIV transmission when initiated >72 hours after exposure. Because the risks associated with antiretroviral medications are likely to outweigh the potential benefit of nPEP in these circumstances, nPEP is not recommended for such exposures, regardless of the HIV status of the source. However, it cannot be concluded on the basis of the available data that nPEP will be completely ineffective when initiated >72 hours after exposure. Moreover, data do not indicate an absolute time after exposure beyond which nPEP will not be effective. When safer and more tolerable drugs are used, the risk-benefit ratio of providing nPEP >72 hours postexposure is more favorable. Therefore, clinicians might consider prescribing nPEP after exposures that confer a serious risk for transmission, even if the exposed person seeks care >72 hours postexposure if, in the clinician's judgment, the diminished potential benefit of nPEP outweighs the potential risk for adverse events from antiretroviral drugs.
Considerations for All Patients Treated with Antiretroviral nPEP
Use of Starter Packs
Patients might be under considerable emotional stress when seeking care after a potential HIV exposure and might not attend to, or retain, all the information relevant to making a decision about nPEP. Clinicians should give an initial prescription for 3--5 days of medication and schedule a follow-up visit to review the results of baseline HIV testing (if rapid tests are not used), provide additional counseling and support, assess medication side effects and adherence, and provide additional medication if appropriate (with an altered regimen if indicated by side effects or laboratory test results).
Scientific Consultation
When clinicians are not experienced with using HAART or when information from source-persons indicates the possibility of antiretroviral resistance, consultation with infectious disease or other HIV-care specialists, if it is available immediately, might be warranted before prescribing nPEP. Similarly, when considering prescribing nPEP to pregnant women or children, consultation with obstetricians or pediatricians might be advisable. However, if such consultation is not immediately available, initiation of nPEP should not be delayed. An initial nPEP regimen should be started and, if necessary, revised after consultation is obtained. Patients who seek nPEP might benefit from referral for psychological counseling that helps ease the anxiety about possible HIV exposure, strengthens risk-reduction behaviors, and promotes adherence to nPEP regimens if prescribed.
Facilitating Adherence
Adherence to antiretroviral medications can be challenging, even for 28 days. In addition to common side effects such as nausea and fatigue, each dose reminds the patient of his or her risk for acquiring HIV infection. Adherence has been reported to be especially poor among sexual assault survivors (92--96). Steps to maximize medication adherence include prescribing medications with fewer doses and fewer pills per dose, educating patients about the importance of adherence and about potential side effects, offering ancillary medications for side effects (e.g., anti-emetics) if they occur, and providing access to ongoing encouragement and consultation by phone or office visit.
Follow-up Testing and Care
All patients seeking care after HIV exposure should be tested for the presence of HIV antibodies at baseline and at 4--6 weeks, 3 months, and 6 months after exposure to determine whether HIV infection has occurred. In addition, testing for sexually transmitted diseases, hepatitis B and C, and pregnancy should be offered (Table 4).
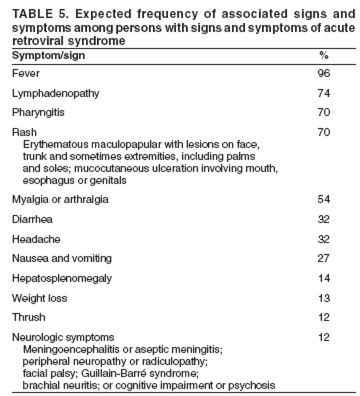
Patients should be instructed about the signs and symptoms associated with acute retroviral infection (Table 5), especially fever and rash (97), and asked to return for evaluation if these occur during or after nPEP. Acute HIV infection is associated with high viral loads. However, clinicians should be aware that available assays might yield low viral-load results (e.g., <3,000) in noninfected persons. Such false-positive results can lead to misdiagnosis of HIV infection (98).
Transient, low-grade viremia has been observed both in macaques exposed to SIV (99) and humans exposed to HIV who were administered antiretroviral PEP (100) and did not become infected. In certain cases, this outcome might represent aborted infection rather than false-positive test results, but this can be determined only through further study. For patients with clinical or laboratory evidence of acute HIV infection, continuing antiretroviral therapy for >28 days might be prudent because such early treatment (no longer prophylaxis) might slow the progression of HIV disease (101). Patients with acute HIV infection should be transferred to the care of HIV treatment specialists.
In addition, clinicians who provide nPEP should monitor liver function, renal function, and hematologic parameters as indicated by the prescribing information found in antiretroviral treatment guidelines (87,102,103), package inserts, and the Physician's Desk Reference (Table 3). Unusual or severe toxicities from antiretroviral drugs should be reported to the manufacturer or FDA.
HIV Prevention Counseling
The majority of persons who seek care after a possible HIV exposure do so because of failure to initiate or maintain effective risk-reduction behaviors. Notable exceptions are sexual assault survivors and children with community-acquired needlestick injuries.
Although nPEP might reduce the risk for HIV infection, it is not believed to be 100% effective. Therefore, patients should practice protective behaviors with sex partners (e.g., abstinence or consistent use of male condoms) or drug-use partners (e.g., avoidance of shared injection equipment) throughout the course of nPEP to avoid transmission to others if they become infected, and after nPEP to avoid future HIV exposures.
At follow-up visits, clinicians should assess their patients' needs for behavioral intervention, education, and services. This assessment should include frank, nonjudgmental questions about sexual behaviors, alcohol use, and illicit drug use. Clinicians should help patients identify ongoing risk issues and develop plans for improving their use of protective behaviors (104).
To help patients obtain indicated interventions and services, clinicians should be aware of local resources for high-quality HIV education and ongoing behavioral risk reduction, counseling and support, inpatient and outpatient alcohol and drug-treatment services, substance/drug abuse treatment programs, family and mental health counseling services, and support programs for HIV-infected persons. Information about publicly funded HIV prevention programs can be obtained from state or local health departments.
Management of Source Persons
When source-persons are seen during the course of evaluating a patient for potential HIV exposure, clinicians should also assess the source-person's access to relevant medical care, behavioral intervention, and social support services. If needed care cannot be provided directly, clinicians should help source-persons obtain care in the community.
If a new diagnosis of HIV infection is made or evidence of other sexually transmitted infection is identified, the patient should be assisted in notifying their sexual and drug-use contacts. Assistance with confidential partner notification (without revealing the patient's identity) is available through local health departments.
Reporting and Confidentiality
Because of the emotional, social, and potential financial consequences of possible HIV infection, clinicians should handle nPEP evaluations with the highest level of confidentiality. Confidential reporting of sexually transmitted infections and newly diagnosed HIV infections to health departments should take place as dictated by local law and regulations.
For cases of sexual assault, clinicians should document their findings and assist patients with notifying local authorities. HIV test results should be recorded separately from the findings of the sexual assault examination to protect patients' confidentiality in the event that medical records are later released for legal proceedings. Certain states and localities have special programs to provide reimbursement for medical therapy, including antiretroviral medication after sexual assault, and these areas might have specific reporting requirements. When the sexual abuse of a child is suspected or documented, the clinician should report it in compliance with state and local law and regulations.
Considerations for Vulnerable Populations
Pregnant Women and Women of Childbearing Potential
Considerable experience has been gained in recent years in the safe and appropriate use of antiretroviral medications during pregnancy, either for the benefit of the HIV-infected woman's health or to prevent transmission to newborns. To facilitate the selection of antiretroviral medications likely to be both effective and safe for the developing fetus, clinicians should consult DHHS guidelines (102) before prescribing nPEP for a woman who is or might be pregnant.
Because of potential teratogenicity, efavirenz should not be used in any nPEP regimen during pregnancy or among women of childbearing age at risk for becoming pregnant during the course of antiretroviral prophylaxis (Table 3). A protease inhibitor- or nucleoside reverse transcriptase inhibitor-based regimen should be considered in these circumstances. When efavirenz is prescribed to women of childbearing potential, they should be instructed about the need to avoid pregnancy. Because the effect of efavirenz on hormonal contraception is unknown, women using such contraception should be informed of the need to use an additional method (e.g., barrier contraception). In addition, because of reports of maternal and fetal mortality attributed to lactic acidosis associated with prolonged use of d4T in combination with ddI in HIV-infected pregnant women, this combination is not recommended for use in an nPEP regimen (105).
Children
Potential HIV exposures in children occur most often by accident (e.g., needlesticks in the community, fights, or playground incidents resulting in bleeding by an HIV-infected child) or by sexual abuse or assaults (106). In a review of charts from 1 year in the pediatric emergency department of one hospital, 10 children considered for nPEP were identified (six because of sexual assault and four because of needlestick injury). Eight began taking nPEP, but only two completed the 4-week course (63,107). An analysis of 9,136 reported acquired immunodeficiency syndrome cases in children identified 26 who were sexually abused with confirmed or suspected exposure to HIV infection (108).
The American Academy of Pediatrics has issued nPEP guidelines for pediatric patients (109). In addition, DHHS pediatric antiretroviral treatment guidelines (103) provide information about the use of antiretroviral agents in children. For young children who cannot swallow capsules or tablets and to ensure appropriate dosing for drugs that do not have capsule/tablet formulations that allow pediatric dosing, drugs for which pediatric formulations are available might need to be prescribed (Table 3). Adherence to the prescribed medications will depend on the involvement of, and support provided to, parents or guardians.
Sexual Assault Survivors
Use of nPEP for sexual assault survivors has been widely encouraged both in the United States and elsewhere (56, 94,110,111). Sexual assault is relatively common among women: 13% of a national sample of adult women reported having ever been raped (60% before age 18), and 5% reported having been raped more than once (112). Sexual assault is not uncommon among men. In one series from an emergency department, 5% of reported rapes involved men sexually assaulted by men (113). Males accounted for 11.6 % of rapes reported among persons aged >12 years who responded to the National Crime Victimization Survey in 1999 (114). However, only three documented cases of HIV infection resulting from sexual assault have been published (94,115,116). In observational studies, HIV infections have been temporally associated with sexual assault (Personal communication, A. Wulfsohn, MD, Sunninghill Hospital, Gauteng, South Africa).
Studies have examined HIV infection rates for sexual assailants (117,118). The largest of these, an evaluation of men incarcerated in Rhode Island, determined that 1% of those convicted of sexual assault were HIV infected when they entered prison, compared with 3% of all prisoners and 0.3% of the general male population (119).
Sexual assault typically has multiple characteristics that increase the risk for HIV transmission if the assailant is infected. In one prospective study of 1,076 sexual assault cases, 20% were attacked by multiple assailants, 39% were assaulted by strangers, 83% of females were vaginally penetrated, and 17% overall were sodomized. Genital trauma was documented in 53% of those assaulted, and sperm or semen was detected in 48% (120). In another study, in which toluidine blue dye was used as an adjunct to naked-eye examination, 40% of assaulted women (70% of nulliparas) had detectable vaginal lacerations, compared with 5% of women examined after consensual sex (121).
Despite these risks and the establishment of multidisciplinary support services, sexual assault survivors often decline nPEP, and many who do take it do not complete the 28-day course. This pattern has been reported in several countries and several programs in North America. In Vancouver, 71 of 258 assault survivors accepted the 5-day starter pack of nPEP, 29 returned for additional doses, and eight completed 4 weeks of therapy (96). Those with the highest risk for HIV exposure (i.e., source known to be HIV infected, a homosexual or bisexual man, or an injection-drug user) were more likely to begin and complete nPEP.
Patients who have been sexually assaulted will benefit from supportive services to improve adherence to nPEP if it is prescribed, and from psychological and other support provided by sexual assault crisis centers. All sexually assaulted patients should be tested and administered prophylaxis for sexually transmitted infections (85), and women who might become pregnant should be offered emergency contraception (122).
Inmates
Certain illegal behaviors that result in imprisonment (e.g., prostitution and injection-drug use) also might be associated with a higher prevalence of HIV infection among prison inmates than among the general population (119). However, studies indicate that the risk for becoming infected in prison is probably less than the risk outside prison (122--125). However, when exposure does occur, because sexual contact and injection-drug use are prohibited in jails and prisons, prisoners who have experienced such exposures might be unable or unwilling to report the behaviors to health-care providers.
Administrators and health-care providers working in correctional settings should develop and implement systems to make HIV education and risk-reduction counseling, nPEP, voluntary HIV testing, and HIV care confidentially available to inmates. Such programs will allow inmates to benefit from nPEP when indicated, facilitate treatment services for those with drug addiction, and assist in the identification and treatment of sexual assault survivors.
Injection-Drug Users
A history of injection-drug use should not deter clinicians from prescribing nPEP if the exposure provides an opportunity to reduce the risk for consequent HIV infection. A survey of clinicians serving injection-drug users determined a high degree of willingness to provide nPEP after different types of potential HIV exposure (126).
In judging whether exposures are isolated, episodic, or ongoing, clinicians should consider that persons who continue to engage in risk behaviors (e.g., commercial sex workers or users of illicit drugs) might be practicing risk reduction (e.g., using condoms with every client, not sharing syringes, and using a new sterile syringe for each injection). Therefore, a high-risk exposure might represent an exceptional occurrence for such persons despite their ongoing risk behavior.
Injection-drug users should be assessed for their interest in substance abuse treatment and their knowledge and use of safe injection and sex practices. Patients desiring substance abuse treatment should be referred for such treatment. Persons who continue to inject or who are at risk for relapse to injection-drug use should be instructed in the use of a new sterile syringe for each injection and the importance of avoiding the sharing of injection equipment. In areas where programs are available, health-care providers should refer such patients to appropriate sources of sterile injection equipment.
Conclusion
Accumulated data from animal and human clinical and observational studies demonstrate that antiretroviral therapy initiated as soon as possible within 48--72 hours of sexual, injection-drug--use, and other substantial nonoccupational HIV exposure and continued for 28 days might reduce the likelihood of transmission. Because of these findings, DHHS recommends the prompt initiation of nPEP with HAART when persons seek care within 72 hours after exposure, the source is known to be HIV infected, and the exposure event presents a substantial risk for transmission. When the HIV status of the source is not known and the patient seeks care within 72 hours after exposure, DHHS does not recommend for or against nPEP but encourages clinicians and patients to weigh the risks and benefits on a case-by-case basis. When the transmission risk is negligible or when patients seek care >72 hours after a substantial exposure, nPEP is not recommended; however, clinicians might consider prescribing nPEP for patients who seek care >72 hours after a substantial exposure if, in their judgment, the diminished potential benefit of nPEP outweighs the potential risk for adverse events from antiretroviral medications. These recommendations are intended for the United States and might not apply in other countries.
* In this report, a nonoccupational exposure is any direct mucosal, percutaneous, or intravenous contact with potentially infectious body fluids that occurs outside perinatal or occupational situations (e.g., health-care, sanitation, public safety, or laboratory employment). Potentially infectious body fluids are blood, semen, vaginal secretions, rectal secretions, breast milk or other body fluid that is contaminated with visible blood.
† Information included in these recommendations might not represent Food and Drug Administration (FDA) approval or approved labeling for the particular products or indications in question. Specifically, the terms safe and effective might not be synonymous with the FDA-defined legal standards for product approval.
|
|
| |
| |
|
|
|