| |
Study Finds that Severity of Liver Damage May Predict Risk for Hepatotoxicity on HAART
|
| |
| |
This study found that the stage of liver disease found by liver biopsy may be the best predictor of whether a patient will experience hepatotoxicity (elevated ALT) on HAART
"Influence of Liver Fibrosis on HAART--Associated Hepatotoxicity in Patients with HIV and Hepatitis C Virus Coinfection"
Clinical Infectious Diseases Feb 15, 2005;40:588-593
Authors: Lidia Aranzabal,1 José L Casado,1 Javier Moya,1 Carmen Quereda,1 Sergio Diz,1 Ana Moreno,1 Leonor Moreno,1 Antonio Antela,1 Maria J. Perez-Elías,1 Fernando Dronda,1 Ana Marín,1 Felix Hernandez-Ranz,2 Alberto Moreno,3 and Santiago Moreno1
Departments of 1Infectious Diseases, 2Gastroenterology, and 3Pathology, Hospital Ramón y Cajal, Madrid, Spain
Key study findings:
--the study reports finding that the severity of liver damage could be the most important factor in explaining the risk of hepatoxicity toxicity, that is whether a patient has early HCV disease or less fibrosis found by liver biopsy; early is F1 or F2 & late stage is F3 or F4. Hepatoxicity is defined in this study as ALT/AST to >5 times the upper limit of normal, or a >3.5-fold increase if baseline levels were abnormal
--study authors report: We have not observed a higher incidence of liver toxicity among patients with mild or moderate fibrosis and with use of nevirapine, efavirenz, or therapies other than NNRTIs, despite the presence of HIV and HCV coinfection or alteration of baseline transaminase levels. But, for patients with later stage HCV disease (F3/F4), there was a higher rate of liver toxicity undergoing NNRTI-based therapy, compared with patients with stage F3 or F4 fibrosis who were not exposed to NNRTIs, (note from Jules Levin: if you look at the table below you can see this analysis is based on a small number of patients.
(note from Jules Levin: these study findings suggest it may be important to evaluate the stage of liver disease with a liver biopsy before starting HAART, because this study suggests the risk for hepatoxicity is greater when the patient has later stage HCV disease. If later stage HCV disease is identified by a liver biopsy, you may decide to treat the HCV before treating HIV. If you do decide to treat HIV first after finding later stage disease by a liver biopsy these study findings suggest it is important to monitor ALT/AST closely after starting HAART & to take note of any development of clinical symptoms of hepatoxicity such as fatigue, nausea, or malaise, jaundice or vomiting).
"...Our study shows that the risk of hepatotoxicity for HIV- and HCV-coinfected patients undergoing treatment depends on the stage of liver fibrosis. Patients with mild or moderate liver fibrosis had an incidence of toxicity of 15%, and we observed a 3-fold increase in the risk of liver toxicity among those with advanced chronic liver disease or cirrhosis. Moreover, as discussed below, the incidence of toxicity was similar for all of the antiretroviral regimens, including NNRTIs or protease inhibitors, used by coinfected patients with mild or moderate fibrosis... There was a higher rate of liver toxicity among patients with stage F3 or F4 liver fibrosis who were undergoing NNRTI-based therapy, compared with patients with stage F3 or F4 fibrosis who were not exposed to NNRTIs... The overall rate of severe hepatotoxicity observed in our coinfected patients (25%) is relatively high, compared with rates reported in previous studies (note from Jules Levin: in this study 38% (18/48) of patients had lat stage HCV disease (stage F3/4)...
... recent data have shown that biopsy continues to be fundamental in establishing the severity of liver damage, especially when the possibility of cirrhosis is present...the correlation of duration of HCV infection and increased ALT values, although weak, could be useful in identifying some patients with advanced chronic liver disease...
...Of greater clinical importance is the lack of correlation between the use of NNRTIs and the emergence of hepatotoxicity in patients with stage F1 or F2 fibrosis. We have not observed a higher incidence of liver toxicity among patients with mild or moderate fibrosis and with use of nevirapine, efavirenz, or therapies other than NNRTIs, despite the presence of HIV and HCV coinfection or alteration of baseline transaminase levels. This fact suggest that the severity of liver damage could be the most important factor in explaining the risk of toxicity for a significant proportion of NNRTI-treated patients, and therefore, this group of patients should be monitored more closely if a decision to initiate treatment with drugs from this class is made. In addition, the importance of the severity of liver damage could explain the contradictory data among different studies when considering the rate of liver toxicity among NNRTI-treated patients [32--34]...
...Risk factors for hepatotoxicity. In a multivariate analysis comparing patients who had stage F3 or F4 fibrosis with patients who had stage F1 or F2 fibrosis, which used hepatotoxicity as the dependent variable, only stage of liver fibrosis (relative risk, 2.75; 95% CI, 1.08--6.97; P = .03) and the baseline ALT value (relative risk, 1.03; 95% CI, 1.01--1.06; P = .04 per each unit of increase) were associated with risk of development of liver toxicity.
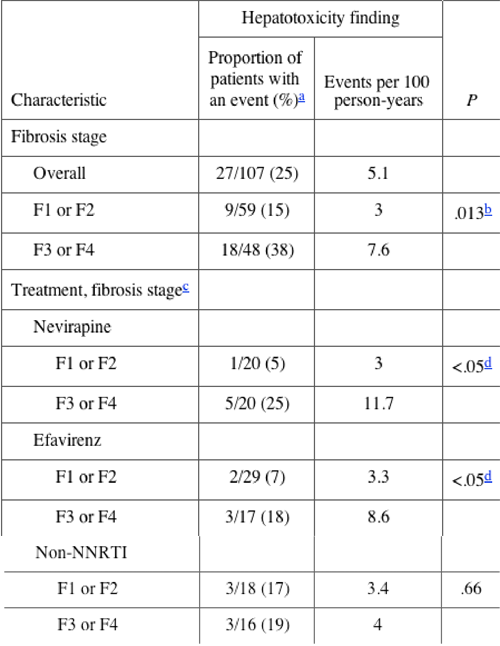
...A significant correlation was observed between liver fibrosis stage and incidence of hepatotoxicity. Thus, 15% of patients (9 of 59) with fibrosis stage F1 or F2 had >1 hepatotoxic event, compared with 38% of patients (18 of 48) with stage F3 or F4...
...Outcome. Overall, 35 episodes of HAART-associated hepatotoxicity were observed in 27 (25%) of 107 patients, with 2 episodes in 4 patients and 3 episodes in 2 patients. Liver toxicity occurred a median of 181 days (95% CI, 32--1100 days) after starting a new antiretroviral therapy. The latter represents an incidence of 5.1 events per 100 person-years of therapy. Only 17 (50%) of the episodes were associated with symptoms, most of which (14 episodes) involved nonspecific complaints such as fatigue, nausea, or malaise. Jaundice or vomiting was observed in 11 (40%) and 6 (23%) of the symptomatic patients, respectively. One patient with cirrhosis died during the episode of liver toxicity, but the remaining 26 patients were cured. Only 10 patients (37%) stopped or changed their antiretroviral treatment. The median CD4+ cell count at the time of appearance of liver toxicity was 464 cells/L and the median HIV load was below detectable limits..."
ABSTRACT
Background. Coinfection with hepatitis C virus (HCV) and human immunodeficiency virus (HIV) is a known risk factor for hepatotoxicity in patients receiving highly active antiretroviral therapy (HAART). The aim of this study was to evaluate the role of HCV-related liver fibrosis in HAART-associated hepatotoxicity. Methods. In a prospective study involving 107 patients who underwent liver biopsy, fibrosis was graded according 5 stages, from F0 (no fibrosis) to F4 (cirrhosis). Hepatotoxicity was defined as an increase in levels of aspartate aminotransferase and alanine aminotransferase to >5 times the upper limit of normal, or a >3.5-fold increase if baseline levels were abnormal. The incidence of hepatotoxicity was compared with liver fibrosis stage and with time and composition of HAART. Results. Overall, 27 patients (25%) had hepatotoxic events (5.1 events/100 person-years of therapy).
The incidence (of hepatoxicity) was greater for patients with stage F3 or F4 fibrosis (38%) than for those with stage F1 or F2 fibrosis (15%; 7.6 vs. 3 events/100 person-years; relative risk, 2.75; 95% confidence interval, 1.08--6.97; P = .013).
Duration of HCV infection, duration of HAART, diagnosis of acquired immunodeficiency syndrome, HCV load, HCV genotype, and nadir CD4+ cell count did not affect the risk of hepatotoxicity.
Of the 86 patients who received nonnucleoside reverse-transcriptase inhibitors (NNRTIs), 11 (13%) developed liver toxicity. In these patients, fibrosis stages F1 and F2 were associated with similar rates of toxicity (3 events/100 person-years for patients who received nevirapine, 3.3 events/100 person-years for those who received efavirenz, and 3.4 events/100 person-years for those who received non-NNRTIs). There was a greater incidence among patients with F3 or F4 fibrosis who received NNRTIs (11.7 events/100 person-years for patients who received nevirapine, and 8.6 events/100 person-years for those who received efavirenz), compared with those who received non-NNRTIs (4 events/100 person-years). Conclusions. HAART-associated hepatotoxicity correlates with liver histological stage in patients coinfected with HIV and HCV. There was no difference in hepatotoxicity risk for different antiretroviral therapies in patients with mild-to-moderate fibrosis.
RESULTS
Population characteristics. A total of 107 patients who underwent liver biopsy between October 2000 and August 2003 were included in the study. Baseline characteristics are summarized in table 1:
median age: 39
79% men
86% IDU HIB risk factor
22% AIDS diagnosis
median nadir CD4 count: 193
median HIV RNA: 2.22 log
alcohol use >60g/day: 21%
median duration of HCV-infection: 17.6 yrs
Median HCV RNA: 1,990,000 copies/ml
HCV genotype 1: 59%; G2: 3%; G3: 24%; G4: 14%
Liver fibrosis score:
F1- 36%
F2- 20%
F3- 32%
F4- 13%
Median ALT at baseline
F1: 78 IU/L
F2: 81
F3: 119
F4: 97
Median AST at baseline:
F1: 72
F2: 97
F3: 106
F4: 98
γ-Glutamyl transminase level, IU/L
F1: 233
F2: 217
F3: 157
F4: 141
Prothrombin activity, INR
F1: 1
F2: 1.02
F3: 1.03
F4: 1.05
The median duration of HCV infection was 17.6 years, and there was a weak but statistically significant correlation between duration of HCV infection and the stage of fibrosis (r = .28; P = .003). The degree of liver fibrosis was not correlated with baseline CD4+ cell count, nadir CD4+ cell count, HIV load, or alcohol abuse, but there was a correlation between fibrosis stage and ALT values (r = .27; P = .005). The relationship among the different variables and the stage of liver fibrosis is shown in table 2: Univariate analysis-HCV genotype p=.04.
Hepatotoxicity in patients receiving NNRTIs. All patients received 3-drug treatment for a median of 5.1 years (95% CI, 2.4--6.8). There was no difference in the duration of HAART between the different histological groups. Overall, 86 patients received NNRTI-based HAART during the study, with no significant association between duration of therapy and severity of chronic liver disease. Thus, 40 patients received nevirapine for a median of 686 days (592 days for patients with fibrosis stages F1 or F2, and 781 days for those with fibrosis stages F3 or F4), and another 46 patients received efavirenz for a median of 758 days (765 days for patients with fibrosis stages F1 or F2, and 745 days for those with fibrosis stages F3 or F4).
Liver toxicity was seen in 11 patients while they were receiving NNRTI-based therapy (6 patients received nevirapine, and 5 received efavirenz). As shown in table 3, there was a higher rate of liver toxicity among patients with stage F3 or F4 liver fibrosis who were undergoing NNRTI-based therapy, compared with patients with stage F3 or F4 fibrosis who were not exposed to NNRTIs. However, the risk of hepatotoxicity for patients with fibrosis stage F1 or F2 was almost identical, regardless of the treatment received. The results remain unchanged when adjusted for CD4+ cell count, HIV load, and duration of HCV infection.
The number of patients in this analysis were small:
Hepatotoxicity findings, according to stage of liver fibrosis and treatment received-
|
|
| |
| |
 |
|
| |
| |
INTRODUCTION
Since the introduction of HAART, HIV infection has become a chronic disease. This has resulted in an increased prevalence and incidence of comorbidities among HIV-infected persons, requiring the use of more medications for longer periods [1]. Therefore, it is not surprising that HAART-associated toxicity, especially liver toxicity, has perhaps become one of the main limitations to treatment [2]. Liver toxicity has been addressed in the context of every antiretroviral regimen, but the risk of hepatotoxic events seems to be higher with nonnucleoside reverse-transcriptase inhibitors (NNRTIs) or ritonavir [3--5].
A substantial proportion of patients with HIV infection have impaired liver function because of coinfection with hepatitis B virus or hepatitis C virus (HCV) [6], the use of hepatotoxic therapies, and the abuse of alcohol and/or other substances [7, 8]. Because HIV and HCV share a route of transmission, coinfection is quite common. This occurs especially among injection drug users and hemophilic patients [6]. Obviously, HCV infection causes chronic liver disease, and moreover, HIV infection accelerates the progression of liver damage to cirrhosis. Therefore, HIV infection may lead to a significant increase in liver-related morbidity and mortality among HCV-infected patients [9]. In addition, coexisting HCV infection could increase the risk of HAART-related hepatotoxicity in HIV-positive patients [10--13].
Controversial data exist concerning the incidence of HAART-related toxicity among patients coinfected with HIV and HCV [13, 14]. The incidence could be affected by different mechanisms of liver injury, the role of immune reactions, and other factors, such as demographic variables [13, 15]. Hypothetically, the degree of liver damage could be a main factor affecting the incidence of HAART-related toxicity among HIV- and HCV-coinfected patients. Thus, the aim of our study was to determine the correlation between HCV-associated liver fibrosis severity and the risk of HAART-associated hepatotoxicity and, specifically, the influence of fibrosis stage on the rate of liver toxicity associated with NNRTI use.
AUTHOR DISCUSSION
Our study shows that the risk of hepatotoxicity for HIV- and HCV-coinfected patients undergoing treatment depends on the stage of liver fibrosis. Patients with mild or moderate liver fibrosis had an incidence of toxicity of 15%, and we observed a 3-fold increase in the risk of liver toxicity among those with advanced chronic liver disease or cirrhosis. Moreover, as discussed below, the incidence of toxicity was similar for all of the antiretroviral regimens, including NNRTIs or protease inhibitors, used by coinfected patients with mild or moderate fibrosis.
However, there is still controversy about the true incidence of liver toxicity among HIV- and HCV-coinfected patients. The overall rate of severe hepatotoxicity observed in our coinfected patients (25%) is relatively high, compared with rates reported in previous studies (7%--13%) [23]. Sulkowski et al. [21] found an incidence of 〜10%, but only 52% of the patients in their study were coinfected with HCV. When HCV- and HIV-coinfected patients were analyzed separately, Sulkowski et al. [21] found an incidence of severe hepatotoxicity of 12% [21]. Law et al. [24] reported similar data in a large study in Thailand, with an incidence of severe hepatotoxicity of 5.8%. However, only 7.6% of the population was confected with HCV and HIV, and a liver toxicity incidence of 12% was described for this subgroup of patients [24]. Of note, these studies were performed during 1996--2001, a time when NNRTIs were scarcely used. Nevertheless, Wit et al. [25] describe a greater overall incidence of liver toxicity (17%), taking into account that only 10.7% of their patients were coinfected with HCV and HIV. This difference between these findings might be explained by the influence of other factors in the development of liver toxicity, such as CD4+ cell count, improvement in immune status, race, and sex [25, 26], and, as confirmed by our results, the impact of preexisting liver damage.
The need for a liver biopsy makes it difficult to ascertain the clinical usefulness of the association between the stage of liver fibrosis and the risk of developing drug-induced hepatotoxicity. Several studies have tried to correlate the severity of histological damage with other more easily measured variables, such as duration of HCV infection and levels of transaminases. However, despite our results, most studies did not find a clear correlation between duration of HCV infection and the stage of liver fibrosis [27--29], probably because of the lack of an established time of HCV infection and the differences in liver disease progression among HIV-infected patients. With respect to transaminases, high levels of AST and ALT are usually associated with liver inflammation and a more advanced stage of fibrosis [27--31], but recent data have shown that biopsy continues to be fundamental in establishing the severity of liver damage, especially when the possibility of cirrhosis is present [30]. However, the correlation of duration of HCV infection and increased ALT values, although weak, could be useful in identifying some patients with advanced chronic liver disease.
Of greater clinical importance is the lack of correlation between the use of NNRTIs and the emergence of hepatotoxicity in patients with stage F1 or F2 fibrosis. We have not observed a higher incidence of liver toxicity among patients with mild or moderate fibrosis and with use of nevirapine, efavirenz, or therapies other than NNRTIs, despite the presence of HIV and HCV coinfection or alteration of baseline transaminase levels. This fact suggest that the severity of liver damage could be the most important factor in explaining the risk of toxicity for a significant proportion of NNRTI-treated patients, and therefore, this group of patients should be monitored more closely if a decision to initiate treatment with drugs from this class is made. In addition, the importance of the severity of liver damage could explain the contradictory data among different studies when considering the rate of liver toxicity among NNRTI-treated patients [32--34].
Our study has several limitations. Because of its observational nature, we cannot exclude a bias in the selection of antiretroviral therapy. Thus, the attending physician could have selected less toxic drugs for this HCV-coinfected population. However, we observed similar durations of HAART among patients with different stages of fibrosis, including the overall time taking NNRTIs. Second, most liver toxicity events were asymptomatic and spontaneously resolved without stopping therapy, suggesting that some hepatotoxic events may not have been diagnosed. Furthermore, our population was selected after biopsy, and a bias toward the inclusion of patients with more advanced HCV infection cannot be excluded. However, the higher incidence of liver toxicity correlated with histological data and not with duration of HCV infection or other clinical data.
In conclusion, our study shows the importance of advanced chronic liver disease in explaining the risk of hepatotoxicity when treating patients coinfected with HCV and HIV. It clarifies controversial data about the incidence of liver toxicity in this population, and of practical importance, it highlights the role of histological damage when potential hepatotoxic drugs are used. Additional studies to address the importance of plasma drug levels and the rate of liver toxicity in this group of patients are ongoing.
PATIENTS AND METHODS
Patients. This study was performed at the HIV Unit of Ramón y Cajal Hospital, a tertiary care hospital in Madrid. It included all HIV- and HCV-coinfected adults who underwent liver biopsy between October 2000 and August 2003 to determine the severity of HCV-associated chronic liver disease. To assess the need for HCV-specific treatment, liver biopsies were requested by the patients' own physicians on the basis of histological findings in the liver, clinical situation, and results of other liver function tests.
The patients underwent follow-up for 2 years after the biopsy date. In addition, clinical and analytical variables had been collected during the 3-year period preceding biopsy, to obtain the maximum information about the course of liver disease and the incidence of liver toxicity associated with HAART. This 3-year period before the biopsy was a conservative choice that was based on studies that showed no histological changes in HCV-associated chronic liver disease 3--6 years before biopsy in HIV-infected patients and for >10 years in non--HIV-infected patients [9, 16, 17].
At the time of the study, all patients were undergoing antiretroviral treatment according to current guidelines. Treatment changes, therapy withdrawal, or any other decision regarding therapy were made by the physician in charge. Patients receiving experimental drugs in the context of clinical trials were not included.
Clinical and laboratory data. Data collected from patients at baseline (i.e., the time of biopsy) included age, sex, risk factors for HIV and HCV infection, past or present diagnosis of opportunistic infections, AIDS diagnosis or HIV infection stage, CD4+ cell count, HIV load, liver function profile, alcohol abuse, or use of other hepatotoxic agents or substances. Results of hepatitis B virus serological analysis, HCV load, and HCV genotype were usually recorded from the beginning of the medical follow-up period. Patients without a known date of HCV infection were considered to have been infected 1 year after starting injection drug use [18] or, in the case of HIV infection associated with other risk factors, on the date on which HIV infection was diagnosed.
An experienced technician performed liver biopsies percutaneously under guidance of ultrasonography. Tru-Cut or Menghini needles were used. Immediate and late complications associated with the liver biopsy were recorded. All liver biopsy specimens >12 mm long were fixed in formalin, embedded in paraffin, and stained with hematoxylin-eosin and Masson's trichrome. They were then analyzed by a single experienced pathologist who was not aware of the clinical and biological data or of the clinical diagnosis made by the attending physician. Histological findings were scored by use of the histological activity index, developed by Knodell et al. [19]. This included grading of necroinflammatory activity and staging of the disease. Fibrosis was separately graded according to the following stages: F0, absent; F1, portal fibrosis; F2, portal-to-portal fibrosis; F3, portal-central bridging; and F4, cirrhosis [20].
In addition, at each subsequent clinical visit, a clinical assessment and physical examination were done, and laboratory data were collected from each patient. Standard laboratory testing included complete blood cell count, CD4+ and CD8+ cell counts, serum biochemistry analyses, and measurements of alanine aminotransferase (ALT) level, aspartate aminotransferase (AST) level, γ-glutamyl aminotransferase level, total bilirubin level, and plasma HIV load. CD4+ cells were counted by flow cytometry. HIV RNA level was determined by branched-DNA assay (Bayer Diagnostics) with a lower limit of detection of 50 copies/mL.
Outcome. Hepatotoxicity was defined in accordance with Sulkowski et al. [21], who used a modified version of the AIDS Clinical Trials Group definition [22]. For patients with normal baseline AST and ALT levels, severe hepatotoxicity was defined as an increase of >5.1 to 10 times the upper limit of normal (severity grades 3--4). In those patients with elevated baseline enzyme levels, hepatotoxicity was defined as an elevation of >3.5 times the upper limit of normal.
Statistical analysis. The baseline values for all analyses were those recorded on the date of liver biopsy. A person-years analysis was used to calculate the incidence of hepatotoxic events. Descriptive statistics were expressed as median values and 95% CIs for continuous variables or as frequency counts and percentages for discrete data. For purposes of the analysis and to increase the statistical power of the study, patients were grouped into 2 categories: those with mild or moderate fibrosis (stages F1 or F2) and those with severe fibrosis or cirrhosis (stages F3 or F4).
Univariate analysis of factors associated with liver toxicity was done by the Student's t test and the Mann-Whitney U test for continuous variables with normal and nonnormal distribution, respectively. The χ2 test and Fisher's exact test were used for qualitative variables. The independent effect of each variable was assessed by means of multivariate analysis using a logistic regression model, with hepatotoxicity as the dependent variable. P < .05 was considered to be statistically significant, and all P values were 2-tailed.
REFERENCES
1. Palella FJ Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection: HIV outpatient study investigators. N Engl J Med 1998; 338:853--60. First citation in article | PubMed
2. Carr A, Cooper DA. Adverse effects of antiretroviral therapy. Lancet 2000; 356:1423--30. First citation in article | PubMed
3. Rodríguez-Rosado R, Garcia-Samaniego J, Soriano V. Hepatotoxicity after introduction of highly active antiretroviral therapy. AIDS 1998; 12:1256. First citation in article | PubMed
4. Kontorinis N, Dieterich D. Hepatotoxicity of antiretroviral therapy. AIDS Rev 2003; 5:36--43. First citation in article | PubMed
5. Dieterich DT, Robinson PA, Love J, Stern JO. Drug-induced liver injury associated with the use of nonnucleoside reverse/transcriptase inhibitors. Clin Infect Dis 2004; 38(Suppl):S80--9. First citation in article | Full Text | PubMed
6. Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the adult AIDS clinical trials group. Clin Infect Dis 2002; 34:831--7. First citation in article | Full Text | PubMed
7. Núñez M, Lana R, Mendoza JL, Martín-Carbonero L, Soriano V. Risk factors for severe hepatic injury after introduction of highly active antiretroviral therapy. J Acquir Immune Defic Syndr 2001; 27:426--31. First citation in article | PubMed
8. Orenstein R, Tsogas N. Looking beyond active antiretroviral therapy: drug-related hepatotoxicity in patients with human immunodeficiency virus infections. Pharmacotherapy 2002; 22:1468--78. First citation in article | PubMed
9. Soto B, Sanchez-Quijano A, Rodrigo L, et al. Human immunodeficiency virus infection modifies the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to cirrhosis. J Hepatol 1997; 26:1--5. First citation in article | PubMed
10. Soriano V, García-Samaniego J, Valencia E, Rodríguez-Rosado R, Muñoz F, González-Lahoz J. Impact of chronic liver disease due to hepatitis viruses as cause of hospital admission and death in HIV-infected drug users. Eur J Epidemiol 1999; 15:1--4. First citation in article | PubMed
11. Núñez M, Ríos P, Martín-Carbonero L, Pérez-Olmeda M, González-Lahoz J, Soriano V. Role of hepatitis C virus genotype in the development of severe transaminase elevation after the introduction of antiretroviral therapy. J Acquir Immune Defic Syndr 2002; 30:65--8. First citation in article | PubMed
12. Qurishi N, Kreuzberg C, Luchters G, et al. Effect of antiretroviral therapy on liver-related mortality in patients with HIV and hepatitis C virus coinfection. Lancet 2003; 362:1708--13. First citation in article | PubMed
13. Den Brinker M, Wit FW, Wertheim--van Dillen PM, et al. Hepatitis B and C virus coinfection and the risk of hepatotoxicity of highly active antiretroviral therapy in HIV-1 infection. AIDS 2000; 14:2895--902. First citation in article | PubMed
14. Monforte Ade A, Bugariny R, Pezzotti P, et al. Low frequency of severe hepatotoxicity and association with HCV co-infection in HIV-positive patients treated with HAART. ICONA (Italian Cohort of Naïve for Antiretrovirals) Study Group. J Acquir Immune Defic Syndr 2001; 28:114--23. First citation in article | PubMed
15. John M, Flexman J, French MA. Hepatitis C virus--associated hepatitis following treatment of HIV-infected patients with HIV protease inhibitors: an immune restoration disease? AIDS 1998; 12:2289--93. First citation in article | PubMed
16. Zarski JP, McHutchison J, Bronowicki JP, et al. Rate of natural disease progression in patients with chronic hepatitis C. J Hepatol 2003; 38:307--14. First citation in article | PubMed
17. Martinez-Sierra C, Arizcorreta A, Diaz F, et al. Progression of chronic hepatitis C to liver fibrosis and cirrhosis in patients coinfected with hepatitis C virus and human immunodeficiency virus. Clin Infect Dis 2003; 36:491--8. First citation in article | Full Text | PubMed
18. Thomas DL, Vlahov D, Solomon L, et al. Correlates of hepatitis C virus infection among injection drug users. Medicine (Baltimore) 1995; 74:212--20. First citation in article | PubMed
19. Knodell RG, Ishak KG, Black WC, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1981; 1:431--5. First citation in article | PubMed
20. Poynard T, Ratziu V, Benmanov Y, Di Martino V, Bedossa P, Opolon P. Fibrosis in patients with hepatitis C: detection and significance. Semin Liver Dis 2000; 20:47--55. First citation in article | PubMed
21. Sulkowski MS, Thomas DL, Chaisson RE, Moore RD. Hepatotoxicity associated with antiretroviral therapy in adults infected with human immunodeficiency virus and the role of hepatitis C or B virus infection. JAMA 2000; 283:74--80. First citation in article | PubMed
22. AIDS Clinical Trials Group. Table of grading severity of adult adverse experiences. Rockville, MD: Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, 1996. First citation in article
23. Bonacini M. Liver injury during highly active antiretroviral therapy: the effect of hepatitis C coinfection. Clin Infect Dis 2004; 38(Suppl):S104--8. First citation in article | Full Text | PubMed
24. Law WP, Dore GJ, Duncombe CJ, et al. Risk of severe hepatotoxicity associated with antiretroviral therapy in the HIV-NAT Cohort, Thailand, 1996--2001. AIDS 2003; 17:2191--9. First citation in article | PubMed
25. Wit FW, Weverling GJ, Weel J, Jurriaans S, Lange JM. Incidence of and risk factors for severe hepatotoxicity associated with antiretroviral combination therapy. J Infect Dis 2002; 186:23--31. First citation in article | Full Text | PubMed
26. Puoti M, Torti C, Ripamonti D, et al. Severe hepatotoxicity during combination antiretroviral treatment: incidence, liver histology, outcome. J Acquir Immune Defic Syndr 2003; 32:259--67. First citation in article | PubMed
27. Macias J, Castellano V, Merchante N, et al. Effect of antiretroviral drugs on liver fibrosis in HIV-infected patients with chronic hepatitis C: harmful impact of nevirapine. AIDS 2004; 18:767--74. First citation in article | PubMed
28. Zarski JP, McHutchinson J, Bronowicki JP, et al. rate of natural disease progression in patients with chronic hepatitis C. J Hepatol 2003; 38:307--413. First citation in article | PubMed
29. Rai R, Wilson LE, Astemborski J, et al. Severity and correlates of liver disease in hepatitis C virus infected injection drug users. Hepatology 2002; 35:1247--55. First citation in article | PubMed
30. Quereda C, Moreno S, Moreno L, et al. The role of liver biopsy in the management of chronic hepatitis C in patients infected with the human immunodeficiency virus. Hum Pathol 2004; 35:1083--7. First citation in article | PubMed
31. Giannini E, Risso D, Botta F, et al. Validity and clinical utility of the aspartate aminotransferase--alanine aminotransferase ratio in assessing disease severity and prognosis in patients with hepatitis C virus--related chronic liver disease. Arch Intern Med 2003; 163:218--24. First citation in article | PubMed
32. Martínez E, Blanco JL, Arnáiz JA, et al. Hepatotoxicity in HIV-1--infected patients receiving nevirapine containing antiretroviral therapy. AIDS 2001; 15:1261--8. First citation in article | PubMed
33. Palmon R, Koo BC, Shoulz DA, Dieterich DT. Lack of hepatotoxicity associated with nonnucleoside reverse transcriptase inhibitors. J Acquir Immune Defic Syndr 2002; 29:340--5. First citation in article | PubMed
34. Sulkowsky MS, Thomas DL, Mehta SH, Chaisson RE, Moore RD. Hepatotoxicity associated with nevirapine- or efavirenz-containing antiretroviral therapy: role of hepatitis C and B infections. Hepatology 2002; 35:182--9. First citation in article | PubMed
|
|
| |
| |
|
|
|