| |
NYC Case: Infection with multidrug resistant, dual-tropic HIV-1 and rapid progression to AIDS: a case report
|
| |
| |
The Lancet
March 19 2005
Martin Markowitz and colleagues report a patient in whom fast progression from acute HIV-1 infection to AIDS and the transmission of a multidrug-resistant virus occurred concurrently. The patient had been infected by an HIV-1 strain that was resistant to several types of antiretroviral drugs; infection progressed to symptomatic AIDS in 4-20 months.
An Editorial this week emphasises that prevention of HIV is still the best way to fight the disease, but warns that the "just say no" approach from conservative religious groups in the USA could hinder such progress. Martin Markowitz's study report follows the Editorial.
HIV/AIDS: doing what's right
Editorial
The Lancet March 19, 2005
The fast-track article in this week's issue describes the case of a New York City man who rapidly developed AIDS after being infected with an HIV strain resistant to three of the four major antiretroviral drug classes: nucleoside reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors, and protease inhibitors.
To acquire resistance, a virus typically pays a price--a fitness cost--that makes it less viable and virulent. But in this case the virus seems to have paid no price, for it has a replicative capacity similar to that of wildtype drug-susceptible viruses.
The clinical course of this infection was also remarkable: as best as can be determined the man had progressed to AIDS within 20 months--and perhaps as little as 4 months--of infection.
New York City public-health officials were understandably alarmed. The man reported that he had unprotected anal sex with multiple partners until his health declined. Concerned about the spread of this HIV strain, New York officials held a press conference to inform the public about the case--a decision that elicited surprisingly acerbic comments from many HIV/AIDS researchers.
The importance of this case remains to be determined. New infections with multidrug-resistant HIV strains have been reported before. Rapid progression to AIDS, too, is not uncommon: in cohort studies a small percentage of patients have progressed to AIDS within several years of seroconversion. In the case reported in this week's Lancet, it is not clear whether rapid progression of the disease was due to the virulence of the virus or to a yet undetermined host susceptibility.
To determine whether this virus is the "nightmare virus", as some tabloids described it, will require additional epidemiological investigation to identify others who may be infected with the strain. The decision of the New York public-health officials to alert the public about the case may well speed up this investigation by encouraging sexual contacts of the patient to seek testing.
This case serves as a reminder that HIV remains a frighteningly versatile foe, one that can mutate to escape immune attack or to acquire drug resistance with surprising speed. One lesson to be drawn from this case, therefore, is that despite all the advances that have been made in understanding this virus and all the progress that has been made in developing new drugs, prevention remains the most effective strategy to combat HIV, especially prevention efforts that target high-risk groups, such as men who have sex with men, intravenous drug users, and sex workers and their clients. Past experience has shown that programmes can be developed to engage these groups and reduce their high-risk behaviour. Prevention programmes developed in the early 1980s were very successful in promoting safer sex practices, for example, among men who have sex with men. Needle-exchange programmes have been shown repeatedly to be effective in reducing needle sharing and the incidence of infection. In a Research Letter in this week's online Lancet, Canadian researchers report that drug users going to a controversial medically supervised injection facility in Vancouver, British Columbia, reduced syringe sharing. Condom promotion campaigns, often conducted by sex workers themselves, have achieved remarkable reductions in HIV infection rates among sex workers and their clients.
Unfortunately, in recent years, such programmes have come under increasing attack by groups, especially conservative Christian groups in the USA, which believe that reaching out to high-risk populations only encourages high-risk behaviours. These religious groups instead promote a "just say no" approach to drug use, sex, and other behaviour they deem immoral. The problem, of course, is that in most instances the "just say no" approach has failed to prove effective. Nevertheless, US officials have promoted abstinence-only sex education over more effective comprehensive programmes, have harassed researchers and outreach groups who work with men who have sex with men, have pressured UN agencies to drop needle-exchange programmes, and have insisted that recipients of US funds ascribe to policies opposing prostitution even though the adoption of such policies may well impair their ability to work with sex workers.
In his first term, US President George W Bush said that in the fight against AIDS "we must concentrate our efforts on programs that work, proven best practices". Unfortunately his administration seems to be more interested in imposing its moral view of the world than saving lives, sacrificing others for its ideology instead of doing what's right.
"Infection with multidrug resistant, dual-tropic HIV-1 and rapid progression to AIDS: a case report"
Martin Markowitz, Hiroshi Mohri, Saurabh Mehandru, Anita Shet, Leslie Berry, Roopa Kalyanaraman, Alexandria Kim, Chris Chung, Patrick Jean-Pierre, Amir Horowitz, Melissa La Mar, Terri Wrin, Neil Parkin, Michael Poles, Christos Petropoulos, Michael Mullen, Daniel Boden, David D Ho
Lancet 2005; 365: 1031-38
See Editorial
Aaron Diamond AIDS Research Center, Rockefeller University, 455 First Avenue, 7th Floor, New York, NY 10016, USA (M Markowitz MD, H Mohri MD, S Mehandru MD, A Shet MD, L Berry BS, R Kalyanaraman BS, A Kim BS, C Chung MA, P Jean-Pierre BA, A Horowitz BS, M La Mar BA, M Poles MD, D Boden MD, Prof D D Ho MD); ViroLogic, San Francisco, CA, USA (T Wrin BS, N Parkin PhD, C Petropoulos PhD); New York University, School of Medicine, New York, NY, USA (A Horowitz, M Poles); and Cabrini Medical Center, New York, NY, USA (M Mullen MD)
Summary
Introduction
Methods
Results
Discussion
Summary
Background: Rapid progression to AIDS after acute HIV-1 infection, though uncommon, has been noted, as has the transmission of multidrug resistant viruses. Here, we describe a patient in whom these two factors arose concomitantly and assess the effects.
Method:s We did a case study of a patient with HIV-1 seroconversion. We genotyped the virus and host genetic markers by PCR and nucleotide sequencing. To ascertain the drug susceptibility of our patient's HIV-1 we did phenotypic studies with the PhenoSense assay. We assessed viral coreceptor use via syncytium formation in vitro and with a modified PhenoSense assay.
Findings: Our patient seems to have been recently infected by a viral variant of HIV-1 resistant to multiple classes of antiretroviral drugs. Furthermore, his virus population is dual tropic for cells that express CCR5 or CXCR4 coreceptor. The infection has resulted in progression to symptomatic AIDS in 4-20 months.
Interpretation: The intersection of multidrug resistance and rapid development of AIDS in this patient is of concern, especially in view of his case history, which includes high-risk sexual contacts and use of metamfetamine. The public health ramifications of such a case are great.
AUTHOR DISCUSSION
Rapid progression to AIDS after acute HIV-1 infection has been described previously,15-18,21,22 as has the transmission of multidrug resistant viruses.4-8,38-42 The unique feature in this case is the convergence of two uncommon factors: the transmission of a multidrug resistant HIV-1 variant and the extremely rapid clinical course to AIDS. The duration of infection in this case cannot have been longer than 20 months, since the patient had five negative HIV-1 antibody tests and normal absolute lymphocyte counts in the period before May, 2003. The transient febrile illness in early November, 2004, arising about 2 weeks after a series of high-risk sexual contacts with multiple partners, could have been the manifestation of his primary HIV-1 infection. If this was the case, then the duration of his infection would be 4-5 months, characterising it as recent but not acute. That the detuned antibody test was positive is in line with an infection beyond the acute phase.23 Likewise, the relative sequence homogeneity in gag p17 and env gp120 V3 is consistent with, although not diagnostic of, recent infection.12,33,34 Thus, our patient has probably been infected for 4-20 months.
The patient has been symptomatic with severe fatigue and weight loss, and his CD4 T-cell counts have been consistently below 80 cells per µL. A diagnosis of AIDS is, therefore, appropriate. However, is his rate of deterioration noteworthy? Review of research done suggests it is. An analysis of the data generated on acute seroconvertors in the Multicenter AIDS Cohort Study, for example, suggests the likelihood of progression to AIDS (CD4 count <200) in 6 and 12 months is seven per 10 000 and 45 per 10 000 individuals, respectively.43 Thus, by comparison, the index case would be in the top 0á5 percentile in terms of rapidity of disease if we assume 12 months as the duration of his infection. Furthermore, an initial analysis of the database in the National Institutes of Health Acute Infection and Early Disease Research Program revealed only six of 1709 cases with persistently low CD4 cell counts. Finally, among about 2700 seroconvertors in the US Military Cohorts, only 15 progressed to clinical AIDS by 1 year of infection.44
Could the rapid clinical course seen in our patient be explained by the properties of his unique HIV-1 variant? The presence of CXCR4-tropic or dual-tropic variants of HIV-1 is associated with a more aggressive clinical course,9 and our patient has a mixture of CCR5-tropic and dual-tropic HIV-1 populations. Despite the presence of a multitude of drug resistance mutations, this virus grows well in vitro and has a greater replication capacity than many wildtype viruses. These in-vitro characteristics, coupled with the great depletion of CXCR4+ T-cell populations in vivo raise the spectre that this strain of HIV-1 might be especially aggressive. That said, our genetic studies on this case are ongoing, and our knowledge of host determinants of rapid disease progression is incomplete. Thus, the cause of the observed clinical course in this man remains unclear.
Treatment options for our patient are limited. His virus is resistant to all protease inhibitors and nevirapine, and is sensitive to efurvitide and efavirenz. The phenotype data for NRTI show susceptibility to various drugs in this class. However, viral mixtures with aminoacid substitutions at positions 184 (conferring resistance to lamivudine and emtricitabine) and with thymidine analogue mutations at 210 and 215 (conferring resistance to abacavir and thymidine analogues) suggests that most NRTIs are unlikely to be effective in vivo.28 Furthermore, the presence of M41L together with mixtures reflected at positions 210 and 219 in RT predicts an attenuated response to tenofovir.45 We believe this case would be difficult to treat with a standard antiretroviral regimen. Efurvitide and efavirenz are the only two antiretroviral drugs that can provide full activity against the virus in this patient. Because of his low CD4 T-cell counts and high viral load, a multidrug regimen, including efurvitide and efavirenz, has been initiated.
Another concern is this man's history of a large number of high-risk sexual contacts and metamfetamine use. Convergence of his sexual history with the multidrug resistant and rapidly-progressing nature of his illness led us to bring this case to the special attention of the New York City Department of Health and Mental Hygiene. Because of the public-health ramifications, they issued a health alert to physicians in the area on Feb 11, 2005.46 Furthermore, tracing of this patient's sexual contacts has begun. Only additional investigations will reveal whether this case is isolated or not. Irrespective of the outcome, efforts to prevent HIV-1 transmission need to be intensified, with particular emphasis on the epidemic that is being propelled by the use of metamfetamine. However, in so doing, care should be taken to avoid punitive measures against the populations most vulnerable to HIV-1.
Contributors
The case was initially seen by M Mullen. M Markowitz identified the case and planned the clinical and laboratory assessments in collaboration with D D Ho, D Boden, A Shet, and H Mohri. L Berry ascertained the genotype. Phenotype, confirmatory genotype, replication capacity, and tropism testing were done in the clinical reference laboratory at ViroLogic under the direction and supervision of T Wrin, N Parkin, and C Petropoulos. Quantitative assays for HIV-1 RNA, cell culture, p24 antigen concentrations, and molecular virology studies were done by R Kalyanaraman, A Kim, C Chung, P Jean-Pierre, and A Horowitz. M La Mar coordinated data collection. M Poles did flexible sigmoidoscopy with biopsy and S Mehandru undertook the flow cytometry studies on blood and gut cells. M Markowitz and D D Ho wrote the report.
Conflict of interest statement
D D Ho has been a paid adviser to ViroLogic since its inception in 1995, and has a brother who is an employee at the company. T Wrin, N Parkin, and C Petropoulos are ViroLogic employees who hold stocks or stock options in the company. No other co-author has a conflict of interest.
Acknowledgments
We acknowledge the efforts of the New York City Department of Health and Mental Hygiene and the nursing staff at the Rockefeller University Hospital. We thank Wendy Chen for preparation of figures and tables; Bayer for provision of genotyping materials; Roche Diagnostics for provision of HIV-1 RNA quantitation materials; L Zhang for contributions to sequence analyses; Z Chen and Y Huang for provision of reagents; J Toma for envelope clonal analysis; T Frieden and G Friedland for comments on the report; and the index case for his cooperation.
This study received funding from the National Institutes of Health (RO1-AI47033, R44-AI048990, and the Acute Infection and Early Disease Research Program, AI41534), Rockefeller University General Clinical Research Center (M01RR00102), and the Columbia-Rockefeller Center for AIDS Research (P30-AI042848).
Introduction
Combination antiretroviral therapy has reduced the rates of death and progression to AIDS in people with HIV-1.1,2 Today, HIV-1 infection can be managed with simple regimens in most infected individuals in developed countries. Along with this therapeutic success, however, has come the emergence of drug resistant HIV-1 in chronically treated patients3 and in some recently infected people.4-8
HIV-1 is classified as either non-syncytium inducing or syncytium inducing,9 and the identification of the chemokine receptors CCR5 and CXCR4 as a necessary entry cofactor has provided a mechanistic explanation for the differences between these virus types;10,11 CCR5-tropic viruses are non-syncytium inducing in phenotype and they dominate in early infection,12 whereas HIV-1 strains that use CXCR4 as a coreceptor are generally syncytium inducing in phenotype and emerge in about half of patients who progress to AIDS.9,13,14 CXCR4-tropic or dual-tropic variants are uncommon in newly infected individuals, but their existence is well documented.15 In fact, transmission of CXCR4-tropic viruses has been reported in individuals who are homozygous for a non-functional CCR5 gene.16-18
The natural history of HIV-1 infection varies widely between hosts, and can be affected by both viral19 and host20 factors. On average, development of AIDS and eventual death arise after years of infection.21 However, cases of rapid progression to immunodeficiency have been reported.15-18,21,22 In some of these patients, CXCR4-tropic or dual-tropic viruses were identified at the outset of infection, suggesting that such viral strains might result in a faster clinical course. It is noteworthy, however, that genetic markers were not adequately analysed in all these cases and cannot therefore be excluded as contributory factors.
Here, we report and analyse a case of infection with a multidrug resistant, dual-tropic HIV-1 virus that resulted in progression to symptomatic AIDS in 4-20 months.
Methods
Between December, 2004, and February, 2005, we assessed one patient (panel 1)23 with documented HIV-1 seroconversion. The patient provided signed informed consent to do all studies described.
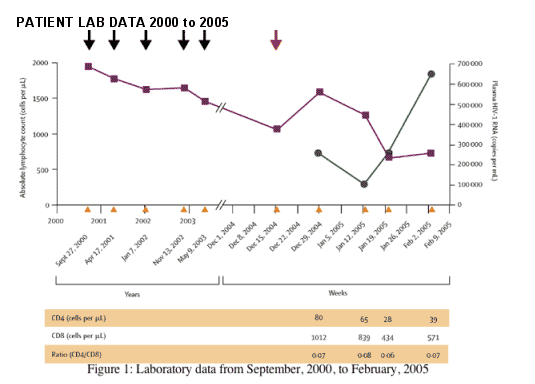
Panel 1: Case report
The patient is a man in his late 40s who has sex with men. He repeatedly tested negative for HIV-1 antibodies between September, 2000, and May, 2003 (figure 1). His absolute lymphocyte counts throughout this period were within the normal range (figure 1). In early November, 2004, he had a fever, pharyngitis, weakness, and fatigue for about a week. These symptoms abated, but intractable sore throat, fatigue, and malaise recurred a few weeks later, prompting a visit to his private doctor in mid-December, 2004. He was diagnosed HIV-1 positive on the basis of the results of an enzyme immunoassay and western blot analysis (all bands positive). On his follow-up visit in late December, 2004, his CD4 T-cell count was 80 cells per µL, his CD8 T-cell count was 1012 cells per µL, and the concentration of HIV-1 RNA in his plasma was 280 000 copies per mL.
He was referred to the Aaron Diamond AIDS Research Center for assessment as a possible case of recent HIV-1 infection. When he was seen in mid-January, 2005, he reported a sore throat, difficulty swallowing, severe fatigue, weight loss, anorexia, and a sense of ill health. His physical examination was normal. Laboratory assessment, however, confirmed the positive HIV-1 serology and a detuned enzyme immunoassay was positive (OD 1á9), indicating his infection was likely beyond the acute or primary phase.23 Several viral load and CD4/CD8 T-cell measurements were taken (figure 1). Collectively, the results suggested that this man had already progressed to symptomatic AIDS with profound CD4 T-cell depletion.
In view of the rapid course seen in this patient, we took a more detailed history. When asked, he reported that he had been sexually active with many male partners over the years, often in conjunction with metamfetamine use. In particular, he believed he was infected while having risky sex with multiple partners in the third week of October, 2004. The patient last took metamfetamine in November, 2004, but continued to have sex with about ten partners until the end of December when sexual activities ceased due to his deteriorating health. He had not taken antiretroviral drugs before his referral to our centre.
|
|
 |
| |
Black arrows=HIV-1 negative serology. Red arrows=HIV-1 positive serology. Yellow arrows=test dates. Unbroken line with closed squares=absolute lymphocyte counts. Unbroken line with closed circles=HIV-1 RNA concentrations.
To assess the susceptibility of our patient's HIV-1 to antiretroviral drugs, we sequenced the viral pol gene and the gp41 envelope open reading frame (Trugene, Bayer Diagnostics, Tarrytown, NY, USA) in a plasma sample from mid-January, 2005. We also did phenotyping studies with the PhenoSense assay (ViroLogic, San Francisco, CA, USA) to ascertain the drug susceptibility of the patient's HIV-1 as well as its replication capacity.24,25
We isolated HIV-1 from the patient's peripheral blood mononuclear cells with a standard protocol,12 and assessed HIV-1 coreceptor use by syncytium induction in MT-2 cells in vitro9 and with a modified PhenoSense tropism assay (ViroLogic).26 We amplified the envelope gene from the patient's plasma virus by RT-PCR, and used it to generate pseudovirions for analysis of viral tropism in U87 cells expressing either CCR5 or CXCR4. We also did analyses of coreceptor use on 14 envelope clones amplified from the plasma of our patient.
In early February, 2004, the patient underwent flexible sigmoidoscopy with biopsy to assess the effect of his infection on the mucosal mononuclear cell population compared with peripheral blood mononuclear cells. We stained these cells with select monoclonal antibodies and analysed them by flow cytometry.
We characterised the genomic diversity of HIV-1 in this case by extracting DNA from our patient's peripheral blood mononuclear cells and undertaking limiting-dilution PCR to amplify a region of gag p17 (nucleotides 814-1279) and env V3 (nucleotides 7032-7336). We sequenced ten clones for every DNA fragment.
We did phylogenetic analysis of a nucleotide sequence from the viral pol gene of the patient and from 30 newly infected individuals identified in 2004 and five reference HIV-1 strains with Clustal X (v1á83).27
The rapid development of AIDS in this patient also prompted us to examine the possibility that he has a genetic predisposition to accelerated disease progression. His HLA Class I and Class II genotypes were identified in a clinically approved laboratory. CCR5 genotype was ascertained by PCR and nucleotide sequencing.
Role of the funding source
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
The results of genotyping studies to ascertain the drug susceptibility of our patient's HIV-1 are summarised in panel 2 and reveal broad resistance to nucleoside reverse transcriptase inhibitors (NRTI), non-nucleoside reverse transcriptase inhibitors (NNRTI), and protease inhibitors. The genotype was confirmed by further testing done at ViroLogic with one notable difference: the detection of a mixture of M184V/I in reverse transcriptase (RT). We interpret the collection of mutations to confer resistance to thymidine analogues, lamivudine and emtricitabine, reduced susceptibility to abacavir and tenofovir,28 high-level resistance to nevirapine,29 possibly an attenuated response to efavirenz,30 and broad resistance to protease inhibitors.31
Panel 2: Genotyping results
Virus*
Genotype
NRTI
M41L A
D67D/N
V118I
M184V/I
L210L/G/M/R/V/W
T215C/Y
K219E
NNRTI
K101E
Y181I
Protease inhibitors no evidence of Delta32 deletion
L10I
L33F
E34Q
M46I
I54M
L63P
A71V
G73S
V77I
I84V
L89V
L90M
*First capital letter denotes aminoacid in wildtype virus; number refers to aminoacid position in gene; and second capital letter indicates aminoacid in virus.
Findings of the phenotyping assay24 indicate that susceptibility (fold change in IC50) of the patient's virus was comparable to a drug-susceptible reference virus for various drugs: abacavir 0á81, didanosine 0á82, stavudine 0á98, zidovudine 0á50, and tenofovir 0á38. We noted low degrees of reduced susceptibility to lamivudine and emtricitabine (3á27-fold and 3á83-fold, respectively). Superficially, these findings suggest little evidence of drug resistance to these agents. However, given the presence of mixtures of viral species detected at aminoacid positions 184, 210, and 215 in RT (panel 2)--all resistance-conferring substitutions for NRTI--a discordance between the genotype and phenotype results is predicted. The minor drug resistant HIV-1 species might not have been scored in the phenotype assay, though they will probably be important in vivo when drugs are administered. Additionally, the results of the phenotyping assay showed the virus was highly resistant to nevirapine and all commercially available protease inhibitors (examples shown in figure 2). The virus tested sensitive to two NNRTI, efavirenz and delavirdine, and to enfurvitide, an inhibitor that blocks HIV-1 entry into cells (figure 2).
Figure 2: Drug susceptibility phenotype to selected agents
|
|
 |
| |
The replication capacity of our patient's HIV-1 was 136%, compared with a median of 100% derived from a large number of wildtype viruses. This finding indicates that, as measured in an in-vitro assay,25 this multidrug resistant virus replicates as well as most wildtype drug-susceptible viruses.
With respect to coreceptor use, although formal kinetic analyses are yet to be done, we can say that this virus replicates to high titres and readily forms syncytia in both MT-2 cells and normal-donor peripheral blood mononuclear cells (figure 3). Formation of the giant cells in an MT-2 culture strongly indicates the presence of CXCR4-tropic viruses.
Data in figure 3 show that the viral quasispecies in this patient are able to infect cells with both CCR5 and CXCR4 coreceptors, indicating that our patient's viruses are, collectively, dual tropic. Furthermore, his viral population consists of CCR5-tropic and dual-tropic viruses in about equal proportions.
Figure 4 shows the results of flow cytometry analyses of peripheral blood mononuclear cells and mucosal mononuclear cells biopsied from our patient in relation to previously published data on uninfected (n=10) and newly infected (n=19) individuals.32 CD4 T-cell depletion in the index case was severe in both peripheral blood mononuclear cells (5%) and mucosal mononuclear cells (1%), and more significant than results seen in other newly infected patients. Furthermore, the depletion is most prominent in subpopulations of cells that express either CXCR4 alone or CXCR4 in conjunction with CCR5 (figure 4). Taken together, these results document the severe depletion of CD4 T cells in our patient's gastrointestinal tract as well as in his blood. Moreover, the great loss of CXCR4+ T cells from the blood and the gut is not only striking, but also suggestive of the functional dominance of CXCR4-tropic viral variants in vivo in this case.
The findings of the viral sequence analysis indicate that the virus in this case belongs to subtype B. The average intrasample diversity for the p17 sequences was small (0á4%) and slightly higher for the V3 sequences (1á7%). The observed relative homogeneity of the viral population is consistent with early HIV-1 infection.12,33,34 However, this sort of genetic data cannot be used easily to establish when a patient became infected by the virus.
We therefore subjected a nucleotide sequence from the viral pol gene of the patient and from 30 newly infected individuals and five reference HIV-1 strains to phylogenetic analysis. Figure 5 shows that the viral sequence of our case is unique, thus eliminating the possibility of contamination. In fact, a search of our sequence database did not yield a match. Because of its unique features, this pol sequence is now being compared with those in the database at the Los Alamos National Laboratory and in various commercial laboratories with the hope of finding a closely related HIV-1 that might provide an epidemiological link to this case.
Panel 2 lists our patient's HLA Class I and Class II genotypes. We did not find HLA alleles that have been associated with rapid progression, including A*24, B*35 Px, B*37, B*56, B*58S, and A1-B8-DR3.35 Additionally, HLA homozygosity is associated with a poor prognosis in HIV-1 infection,35 but was not observed in this case. A larger panel of disease-accelerating genetic markers is now being examined.20
We also studied the possibility that this patient acquired a CXCR4-tropic virus because he is CCR5-/- because of the Delta32 mutation.36,37 This possibility was excluded by the absence of a Delta32 allele (panel 2) and by positive staining of CCR5 in the patient's peripheral blood and mucosal mononuclear cells.
References
1 Egger M, May M, Chene G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet 2002; 360: 119-29. [Text]
2 Palella FJ Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med 1998; 338: 853-60. [PubMed]
3 Richman DD, Morton SC, Wrin T, et al. The prevalence of antiretroviral drug resistance in the United States. AIDS 2004; 18: 1393-401. [PubMed]
4 Hecht FM, Grant RM, Petropoulos CJ, et al. Sexual transmission of an HIV-1 variant resistant to multiple reverse-transcriptase and protease inhibitors. N Engl J Med 1998; 339: 307-11. [PubMed]
5 Little SJ, Holte S, Routy JP, et al. Antiretroviral-drug resistance among patients recently infected with HIV. N Engl J Med 2002; 347: 385-94. [PubMed]
6 Boden D, Hurley A, Zhang L, et al. HIV-1 drug resistance in newly infected individuals. JAMA 1999; 282: 1135-41. [PubMed]
7 Simon V, Vanderhoeven J, Hurley A, et al. Evolving patterns of HIV-1 resistance to antiretroviral agents in newly infected individuals. AIDS 2002; 16: 1511-19. [PubMed]
8 Grant RM, Hecht FM, Warmerdam M, et al. Time trends in primary HIV-1 drug resistance among recently infected persons. JAMA 2002; 288: 181-88. [PubMed]
9 Koot M, Keet IP, Vos AH, et al. Prognostic value of HIV-1 syncytium-inducing phenotype for rate of CD4+ cell depletion and progression to AIDS. Ann Intern Med 1993; 118: 681-88. [PubMed]
10 Dragic T, Litwin V, Allaway GP, et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 1996; 381: 667-73. [PubMed]
11 Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 1996; 272: 872-77. [PubMed]
12 Zhu T, Mo H, Wang N, et al. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science 1993; 261: 1179-81. [PubMed]
13 Richman DD, Bozzette SA. The impact of the syncytium-inducing phenotype of human immunodeficiency virus on disease progression. J Infect Dis 1994; 169: 968-74. [PubMed]
14 Koot M, van Leeuwen R, de Goede RE, et al. Conversion rate towards a syncytium-inducing (SI) phenotype during different stages of human immunodeficiency virus type 1 infection and prognostic value of SI phenotype for survival after AIDS diagnosis. J Infect Dis 1999; 179: 254-58. [PubMed]
15 Yu XF, Wang Z, Vlahov D, Markham RB, Farzadegan H, Margolick JB. Infection with dual-tropic human immunodeficiency virus type 1 variants associated with rapid total T cell decline and disease progression in injection drug users. J Infect Dis 1998; 178: 388-96. [PubMed]
16 Sheppard HW, Celum C, Michael NL, et al. HIV-1 infection in individuals with the CCR5-Delta32/Delta32 genotype: acquisition of syncytium-inducing virus at seroconversion. J Acquir Immune Defic Syndr 2002; 29: 307-13. [PubMed]
17 Balotta C, Bagnarelli P, Violin M, et al. Homozygous delta 32 deletion of the CCR-5 chemokine receptor gene in an HIV-1-infected patient. AIDS 1997; 11: F67-71. [PubMed]
18 Michael NL, Nelson JA, KewalRamani VN, et al. Exclusive and persistent use of the entry coreceptor CXCR4 by human immunodeficiency virus type 1 from a subject homozygous for CCR5 delta32. J Virol 1998; 72: 6040-47. [PubMed]
19 Learmont JC, Geczy AF, Mills J, et al. Immunologic and virologic status after 14 to 18 years of infection with an attenuated strain of HIV-1: a report from the Sydney Blood Bank Cohort. N Engl J Med 1999; 340: 1715-22. [PubMed]
20 Hogan CM, Hammer SM. Host determinants in HIV infection and disease, part 2: genetic factors and implications for antiretroviral therapeutics. Ann Intern Med 2001; 134: 978-96. [PubMed]
21 Munoz A, Wang MC, Bass S, et al. Acquired immunodeficiency syndrome (AIDS)-free time after human immunodeficiency virus type 1 (HIV-1) seroconversion in homosexual men. Am J Epidemiol 1989; 130: 530-39. [PubMed]
22 Keet IP, Krijnen P, Koot M, et al. Predictors of rapid progression to AIDS in HIV-1 seroconverters. AIDS 1993; 7: 51-57. [PubMed]
23 Janssen RS, Satten GA, Stramer SL, et al. New testing strategy to detect early HIV-1 infection for use in incidence estimates and for clinical and prevention purposes. JAMA 1998; 280: 42-48. [PubMed]
24 Petropoulos CJ, Parkin NT, Limoli KL, et al. A novel phenotypic drug susceptibility assay for human immunodeficiency virus type 1. Antimicrob Agents Chemother 2000; 44: 920-28. [PubMed]
25 Barbour JD, Wrin T, Grant RM, et al. Evolution of phenotypic drug susceptibility and viral replication capacity during long-term virologic failure of protease inhibitor therapy in human immunodeficiency virus-infected adults. J Virol 2002; 76: 11104-12. [PubMed]
26 Richman DD, Wrin T, Little SJ, Petropoulos CJ. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc Natl Acad Sci USA 2003; 100: 4144-49. [PubMed]
27 Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 1997; 25: 4876-82. [PubMed]
28 Johnson VA, Brun-Vezinet F, Clotet B, et al. Update of the drug resistance mutations in HIV-1: 2004. Top HIV Med 2004; 12: 119-24. [PubMed]
29 Richman DD, Havlir D, Corbeil J, et al. Nevirapine resistance mutations of human immunodeficiency virus type 1 selected during therapy. J Virol 1994; 68: 1660-66. [PubMed]
30 Bacheler L, Jeffrey S, Hanna G, et al. Genotypic correlates of phenotypic resistance to efavirenz in virus isolates from patients failing nonnucleoside reverse transcriptase inhibitor therapy. J Virol 2001; 75: 4999-5008. [PubMed]
31 Kozal M. Cross-resistance patterns among HIV protease inhibitors. AIDS Patient Care STDS 2004; 18: 199-208. [PubMed]
32 Mehandru S, Poles MA, Tenner-Racz K, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med 2004; 200: 761-70. [PubMed]
33 Zhang LQ, MacKenzie P, Cleland A, Holmes EC, Brown AJ, Simmonds P. Selection for specific sequences in the external envelope protein of human immunodeficiency virus type 1 upon primary infection. J Virol 1993; 67: 3345-56. [PubMed]
34 Ganeshan S, Dickover RE, Korber BT, Bryson YJ, Wolinsky SM. Human immunodeficiency virus type 1 genetic evolution in children with different rates of development of disease. J Virol 1997; 71: 663-77. [PubMed]
35 Carrington M, O'Brien SJ. The influence of HLA genotype on AIDS. Annu Rev Med 2003; 54: 535-51. [PubMed]
36 Dean M, Carrington M, Winkler C, et al. Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Science 1996; 273: 1856-62. [PubMed]
37 Liu R, Paxton WA, Choe S, et al. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 1996; 86: 367-77. [PubMed]
38 Chan KC, Galli RA, Montaner JS, Harrigan PR. Prolonged retention of drug resistance mutations and rapid disease progression in the absence of therapy after primary HIV infection. AIDS 2003; 17: 1256-58. [PubMed
39 Yerly S, Kaiser L, Race E, Bru JP, Clavel F, Perrin L. Transmission of antiretroviral-drug-resistant HIV-1 variants. Lancet 1999; 354: 729-33. [Text]
40 Brenner BG, Routy JP, Petrella M, et al. Persistence and fitness of multidrug-resistant human immunodeficiency virus type 1 acquired in primary infection. J Virol 2002; 76: 1753-61. [PubMed]
41 Colson P, Ravaux I, Yahi N, Tourres C, Gallais H, Tamalet C. Transmission of HIV-1 variants resistant to the three classes of antiretroviral agents: implications for HIV therapy in primary infection. AIDS 2002; 16: 507-09. [PubMed]
42 Kuritzkes DR, Bell S, Bakhtiari M. Rapid CD4+ cell decline after sexual transmission of a zidovudine-resistant syncytium-inducing isolate of HIV-1. AIDS 1994; 8: 1017-19. [PubMed]
43 Gange S. Variations in the natural history of HIV infection in the NIAID Multicenter AIDS Cohort Study (MACS) and the Women's Interagency HIV Study (WIHS). 12th Annual Conference on Retroviruses and Opportunistic Infections, Boston, MA, USA; Feb 22-25, 2005.
44 Dolan M. Variations in the natural history of HIV seroconvertors in US Military Cohorts. 12th Annual Conference on Retroviruses and Opportunistic Infections, Boston, MA, USA; Feb 22-25, 2005.
45 Miller MD, Margot NA, Hertogs K, Larder B, Miller V. Antiviral activity of tenofovir (PMPA) against nucleoside-resistant clinical HIV samples. Nucleosides Nucleotides Nucleic Acids 2001; 20: 1025-28. [PubMed]
46 New York City Department of Health and Mental Hygiene. NYC health alert: New York City resident diagnosed with rare strain of multi-drug resistant HIV that rapidly progresses to AIDS. Feb 11, 2005.
|
|
| |
| |
|
|
|