| |
Treatment of Facial Lipoatrophy With Intradermal Injections of Polylactic Acid in HIV-Infected Patients
|
| |
| |
JAIDS Journal of Acquired Immune Deficiency Syndromes
1 April 2005
Lafaurie, Matthieu MD*; Dolivo, Marc MD*; Porcher, Raphel MD ; Rudant, Jrmie MD ; Madelaine, Isabelle PharmDà; Molina, Jean-Michel MD*
From the *Service des Maladies Infectieuses et Tropicales, Dpartement de Biostatistique et Informatique Mdicales, and àPharmacie Centrale, Hpital Saint-Louis, Paris, France.
Abstract
Background: This study assessed the safety and efficacy of intradermal injections of polylactic acid (PLA) in patients with facial lipoatrophy under antiretroviral therapy.
Methods: In a prospective open-label study, PLA was injected in both cheeks every 2 weeks. The primary efficacy endpoint was the patient's self-perception of improvement as assessed by a visual analogue scale (VAS). Secondary endpoints included 3-dimensional photographs (3DP) to measure dermal thickness, quality of life (QoL) scores, and adverse events.
Results: Ninety-four patients received a median of 5 sets of injection in both cheeks. Median age was 43 years, and median CD4 cell count was 500/mm3. Median VAS score significantly increased from 3.4/10 at baseline to 6.8/10 at the end of the treatment procedure and was sustained at 7/10 at the end of follow-up (P < 0.0001 vs. baseline). Median dermal thickness increase was 2.3 mm at the end of follow-up. QoL scores remained unchanged. Seventeen patients needed further injections of PLA during a median follow-up of 12 months. Injections were well tolerated with only 1 serious adverse event (anaphylactic reaction) that necessitated treatment interruption.
Conclusions: Injections of PLA improved patients' self-perception of facial lipoatrophy, with a good safety profile. The benefit of the procedure, however, decreased with time.
AUTHOR DISCUSSION
The mechanisms of antiretroviral-associated lipodystrophy in patients with HIV-infection remain poorly understood, and therapeutic interventions such as changes in antiretroviral therapy or use of rosiglitazone have provided inconsistent and rather deceptive results, especially regarding the correction of facial lipoatrophy.22-24 This explains why plastic surgery and cosmetic therapy using filling components are still the preferred options in the treatment of facial atrophy, the most obvious and stigmatizing manifestation of lipodystrophy.13,14,25,26 The risks of surgery, the expertise needed to use implants, the impossibility in some patients of performing autologous fatty tissue injection because of insufficient abdominal fat to be transferred into the face, and the lack of long-term safety data make surgery a second-line therapy. A cosmetic approach using filling components seems to be more attractive. Among filling products, PLA has been safely used for about 10 years in orthopedic and facial surgery and for correction of scars and wrinkles.27,28 Its mechanism of action relies on immediate filling due to the volume of the injected material and subsequent increase in dermal thickness through a rise in fibroblast number and the deposition of collagen fibers. PLA offers advantages over the other facial implants as it is hypoallergenic, biodegradable, and bioresorbable and cannot therefore trigger inflammatory reactions. These characteristics have led to the use of PLA for the treatment of HIV-associated lipoatrophy of the face.17-19 Previous reports have shown the good safety profile of this treatment and its efficacy. However, the number of patients treated remained small, the criteria used to assess efficacy varied from study to study, and the long-term benefit and safety of this procedure remained uncertain. We report here our experience with a large number of HIV-infected patients who received PLA injection in the cheeks and temples for the treatment of antiretroviral-associated facial lipoatrophy and assessed the safety and efficacy of the procedure. Ninety-four patients received at least 1 PLA injection and were followed for a median of 12 months. The safety and tolerability of the procedure were good as only 1 patient presented a treatment-limiting serious adverse event (anaphylactic reaction) that resolved completely following treatment discontinuation. This anaphylactic reaction could also be attributed to the other components of the injected material such as mannitol or carboxymethylcellulose.29,30 Pain related to the injection was the most frequent adverse event but was mild to moderate in almost all cases and never led to treatment discontinuation. Postinjection edema was also reported in all patients. This edema was mild, transient, and resolved in 1-3 days. Subcutaneous nodules in the injected area were reported in 12% of patients during follow-up. No cellulitis was reported.
The main efficacy endpoint of our study was the patient self-perception of improvement of his facial lipoatrophy as assessed by a VAS score using a scale specifically designed for the study. Using this subjective criterion, 82% of the patients perceived an improvement of their facial lipoatrophy at the end of treatment as compared with baseline, and this proportion was sustained to up to 76% at the last assessment visit during follow-up. According to our psychologist, this benefit was associated with increased self-confidence in most patients, who were able to resume a normal social life. The severity of the lipoatrophy as judged at baseline by the physician was not associated with a worse outcome. Only a low baseline patient VAS score was associated with a better outcome in a multivariate analysis assessing the role of patient baseline characteristics in the success of the procedure.
These good results, however, should be tempered by the analysis of secondary efficacy endpoints, which underscore the difficulty of the assessment of such a procedure. Indeed, mental and physical QoL scores did not increase significantly after the treatment procedure or during follow-up. This could be explained, in part, by the fact that the MOS SF-36 questionnaire used in this study might not be adequate to accurately address the issue of body appearance. Issues such as self-esteem, sexuality, daily performance, and social life might be interesting to address in future studies. The low scores of QoL reported in our patients are, however, consistent with recent data showing the negative impact of the lipodystrophy syndrome on QoL.31,32 Also the median increase in dermal thickness of the injected areas as assessed by 3DP, although significant, was only in the range of 2 mm after the treatment, which was smaller than the increase reported in other studies using ultrasound. It is, however, difficult to compare results obtained in these different studies, as none of the methods used to measure dermal thickness has been validated. Also, when independent assessors were asked to order DP of patients taken before or after treatment, only 64-76% of the DP sets were correctly ranked, with a low to moderate agreement between observers. These data suggest that either standardized photographs may not represent an ideal mean of assessing the outcome of cosmetic interventions or that objective improvement following PLA injections was small. Indeed, patient's self-assessment of improvement may only represent wishful thinking and may not be fully indicative of an objective benefit. There is indeed a crucial need for an accurate definition and assessment of lipoatrophy, using validated objective tools. Until then, the patient self-perception of lipoatrophy may represent one of the most appropriate criteria.
Finally, 17/86 patients (19.5%) needed reinjections during follow-up. The estimated probability of reinjection (Fig. 2) was 45% at 15 months. This result shows that the benefit of PLA therapy may be short-lived in some patients and that reinjections should be considered in a substantial proportion of patients, 1 year after the beginning of treatment.
In conclusion, our study confirmed that the use of PLA injections in the treatment of facial lipoatrophy is a safe procedure. Also, the benefit of this procedure as assessed by the patient self-perception of improvement (a subjective assessment) persisted for up to 15 months beyond the last injection, a time at which new injections might be necessary for up to 45% of the patients. Further studies on the treatment of facial lipoatrophy with PLA need to be conducted to address issues such as the number of injections to be performed, the volume to be injected, the best interval between injections, and the long-term safety of the procedure.
RESULTS
Patients
Ninety-eight patients were enrolled in the study. Four patients, however, did not start the treatment procedure. One patient suddenly died before the initiation of the therapy, 2 patients withdrew consent, and the dermatologist practicing the injections thought that the facial atrophy of the 4th patient was too mild and did not require any treatment. Ninety-four patients therefore received at least 1 injection of PLA and are the purpose of the study. Baseline characteristics of the patients are summarized in Table 1. Most patients were white homosexual men, with a median age of 44 years. All but 4 patients were on HAART, 42% of them receiving a stavudine-including regimen. Plasma HIV RNA levels were <200 copies/mL in 71% of patients, and median CD4 cell count was 500/mm3.
Table 1: 94% men; median age: 44; HIV risk factors: 57% homosexual; 19% heterosexual; 9% IVDU; 15% unknown.
93% white.
CDC clinical stage:
A 90%
B 6%
C 3%
Median CD4 500 cells
# with HIV RNA <200 c/ml: 71%
HAART
Nrtis only: 5%
PI therapy: 54%
NNRTI therapy: 40%
No treatment: 4%
D4T containing regimen: 42%
SEVERITY of LIPOATROPHY
Mild: 36%
Moderate: 43%
Severe: 21%
PLA Injections
The median number of injections per cheek and per patient was 5 (minimum: 1; maximum: 7). The median volume of PLA injected per session was 2.5 mL (range: 1.0-3.5) and 2.4 mL (range: 1.0-3.8) for the right and left cheek, respectively. The median duration of the treatment procedure (time between the first and the last injection) was 2.3 months (range: 0.5-7). Thirty-nine patients (41%) also required injections in temples. Four patients were lost to follow-up (after 2, 3, 4, and 5 injections, respectively), and the treatment was prematurely discontinued in 3 other patients (after 1, 3, and 5 injections, respectively), because of progression of HIV disease in 2 patients and anaphylactic reaction after the first injection in 1 case. The median follow-up of patients from the time of the first injection was 12 months (1-27), and the median time between the last injection and the last follow-up visit (or first reinjection) was 6 months (1-20).
Safety and Tolerance
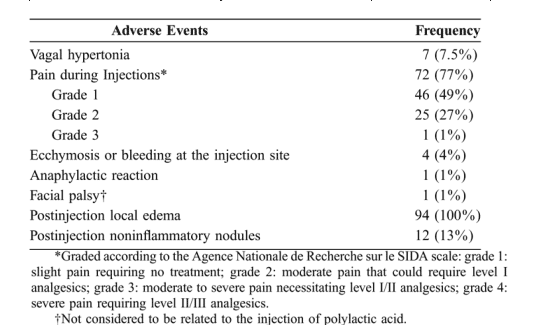
Pain related to the injection was reported in 72 patients (77%) and was graded 1 or 2 in all but one case (grade 3 pain) (Table 2). Malaise, occurring in 7 patients, was noted mainly during the first injection of PLA and was attributed to vagal hypertonia. No treatment was required, and all 7 patients were willing to continue the procedure. Mild bleeding was noted in 3 patients but ceased rapidly after local pressure. A small bruising developed a few minutes after the injection in 1 patient and healed completely after several days without any treatment. In all patients, mild and localized edema of the cheeks developed within hours after each injection and resolved within the next 2-3 days. No cellulitis was observed. In 12 cases, subcutaneous noninflammatory nodules were noted at the injection site, 2-9 months after the first injection. These small (2- to 4-mm) nodules were detected only by digital palpation and were not symptomatic. No biopsy was performed and the natural history of these nodules will require further follow-up.
|
|
 |
| |
Two patient experienced serious adverse events, 1 of which was likely to be treatment related. One patient developed a painful generalized edema associated with inflammatory noninfiltrated maculae on wrists, ankles, and calves 2 days after the first injection of PLA. There were no skin changes at the site of injection. This event was diagnosed as an anaphylactic reaction. The patient received an oral nonsteroidal anti-inflammatory drug, and all signs and symptoms resolved within a few days. Blood tests were normal. No further injection of PLA was performed in this patient. Another patient presented with a peripheral facial palsy 10 days after the 3rd injection of PLA. Brain MRI and cerebrospinal fluid analysis were normal. A treatment with valaciclovir and steroids was initiated and the facial palsy gradually healed within 3 weeks. Because the patient had a history of Bell palsy, he felt this event was not related to PLA and was willing to receive further PLA injections. Since no recurrence of the facial palsy was noted during the next PLA injections, this event was considered not related to the injections of PLA.
Efficacy Endpoints
Self-assessed patient median VAS score for facial lipoatrophy significantly increased from 3.4/10 at baseline to 6.8/10 at the end of the treatment procedure (P < 0.0001). Figure 1 shows the increase in VAS score according to the number of injections received, with a plateau obtained after 3 injections. The success rate defined as the proportion of patients whose VAS scores increased at the end of the treatment procedure as compared with baseline, with patients lost to follow-up and prematurely discontinuing the procedure considered as failures, was 82% (77/94 patients). In a multivariate analysis, patients with success were not different from patients with failure in any baseline characteristic (age, sex, ethnicity, HIV risk factor, Centers for Disease Control clinical stage, CD4 cell count, antiretroviral therapy, severity of the lipoatrophy) except that their VAS score was significantly lower (3.2 as compared with 6.2, P = 0.001). Also, patients with success received a higher median number of injections (5 vs. 4, P = 0.02). At the last follow-up visit, among 87 patients with data available, median VAS score remained significantly higher than at baseline (7/10 as compared with 3.4/10, P < 0.001), and success rate was sustained at 73.5% (69/94 patients).
Three-dimensional photographs demonstrated increase in dermal thickness at the injection sites in both cheeks. Among the 49 patients with 3DP, median increase in dermal thickness was 1.9 mm after the end of treatment as compared with baseline and was sustained at 2.3 mm at the last follow-up visit.
Two independent observers not directly involved in the study, but familiar with lipoatrophy, were asked to correctly order 2 sets of DP of the face for each patient. The first set included DP from 83 patients taken at baseline and at the end of treatment. Both assessors correctly ranked DP for 53 (64%) and 55 (66%) patients, respectively, with a kappa score of 0.26, indicating a low agreement between assessors. The second set included DP from 72 patients taken at baseline and at the last follow-up visit. Both assessors correctly ranked DP for 49 (68%) and 55 (76%) patients, respectively, with a kappa score of 0.38, indicating a moderate to good agreement between assessors.
Mental and physical QoL scores using the SF-36 questionnaires showed no significant changes from baseline when measured at the end of the treatment or at the last follow-up visit.
Reinjections of Polylactic Acid During Follow-up
New injections were performed in 17/87 patients (19.5%) during follow-up. These injections were allowed after a minimum of 3 months from the last injection, when both the patient and the dermatologist felt that the benefit obtained with the first round of injections was lost. In these patients, the median baseline VAS score was low (2.0), increased to 7.8 after the first set of injections, but dropped to 6.1 at the time new injections were performed. The probability of reinjection, 15 months after the end of treatment, was 45% (95% CI: 22-61%) as assessed by the Kaplan-Meier method.
INTRODUCTION
Fat redistribution or lipodystrophy has emerged as a perplexing and frequent complication in HIV-infected patients receiving highly active antiretroviral therapy (HAART).1-3 Lipoatrophy of the face is one of the most serious manifestations of lipodystrophy.4-6 The psychologic and social impact of facial lipoatrophy constitutes a major concern in these patients.7,8 These body changes give an unhealthy aspect that resembles wasting and are a reminder of disease in otherwise healthy HIV-infected patients.9 Furthermore, this side effect of treatment may affect adherence to antiretroviral therapy, which may in turn favor treatment failure.10 As the mechanism of lipodystrophy remains obscure, no clear guidelines for its prevention or treatment have been established.11 Today, treatment of facial atrophy consists of either plastic surgery or filling with synthetic components.12 Among filling components, polylactic acid (PLA) is a biocompatible, biodegradable, and alleged immunologically inert material that is obtained by biosynthesis.13-15 Its injection into the deep dermis stimulates fibroblast multiplication and collagen production. The main objective of the present study was to evaluate the safety and efficacy of intradermal injections of PLA in the treatment of facial lipoatrophy in HIV-infected patients.
METHODS
This study was a prospective, open-label, single-arm study of the safety and efficacy of PLA intradermal injections in the treatment of lipoatrophy in HIV-infected patients and was conducted in a tertiary university hospital (Saint-Louis Hospital in Paris, France).
Study Population
From September 2001 to December 2002, adult HIV-infected patients with antiretroviral therapy-induced facial lipoatrophy were prospectively enrolled in this study. Main inclusion criteria were adult with HIV infection; facial lipoatrophy; no current opportunistic infection; and sustained control of HIV infection with a CD4 cell count ³200/mm3 and stable antiretroviral therapy for at least 3 months. Patients were excluded if they had one of the following criteria: abnormal coagulation tests; concomitant therapy with nonsteroidal anti-inflammatory drugs or acetyl salicylic acid; skin disease of the face; pregnancy or breast-feeding; major or unstable concomitant illness. Patients with a history of surgical or cosmetic intervention for facial lipoatrophy were also excluded.
All patients had a screening visit, 1 month before the first injection, with the same dermatologist who would perform the injections. Lipoatrophy was classified into 3 categories (adapted from James et al16): mild (localized and mild facial lipoatrophy with an almost normal appearance), moderate (deeper and wider central cheek atrophy, with the facial muscles showing through), and severe (extends up toward the orbit; the facial skin lies directly on the muscles over a wide area). All patients provided written, informed consent. Support by a psychologist was offered throughout the treatment and follow-up.
PLA Injections
Injections of PLA (New-Fill, Dermik Laboratories, Berwyn, PA, a division of Aventis, Strasbourg, France) were performed in the median-deep layer of the dermis by the same experienced dermatologist according to the manufacturer's instructions. Briefly, PLA was reconstituted from sterile dry powder of 0.15 g of PLA by the addition of 3 mL of sterile water. PLA was mixed with 1 mL of adrenaline-free lidocaine, and the preparation was divided in 4 syringes of 1 mL and injected into the deep dermis of the affected area, using 26 G ~ 1/2 needles. At each session, patients received several intradermal injections in each cheek. After the injections, ice was applied for 10 minutes to reduce the risk of hematoma and edema. Then, a thorough and prolonged massage was performed, to homogenize the distribution of the injected product and to prevent the development of dermal nodules at the site of injection. Injections were made into both cheeks at each visit, every 2 weeks. Injections in temples were also practiced when judged necessary. The number of sets of injections to be performed was left to the patients' and dermatologist's discretion, but according to earlier reports, a minimum of 3 sets of injections was thought necessary to obtain a satisfactory result.17-19 After the completion of the treatment procedure, patients were assessed by the same dermatologist on a monthly basis. New injections of PLA were allowed during follow-up but could not be performed in the first 3 months following the last injection. Patients' follow-up was censored at the time of reinjection.
Safety and Tolerance
The safety of the treatment was assessed, at each visit, through clinical examination. The severity of adverse events was graded according to the Agence Nationale de Recherche sur le SIDA (ANRS) grading scale. The pain related to the injections was graded according to its severity as follows: grade 1, slight pain requiring no treatment; grade 2, moderate pain that could require level I analgesics; grade 3, moderate to severe pain necessitating level I/II analgesics; and grade 4, severe pain requiring level II/III analgesics.
Assessment of the Efficacy of Treatment
The primary efficacy endpoint was the patient's subjective self-perception of improvement of facial lipoatrophy as assessed by a visual analogue scale (VAS). Patients were asked to record, on a 10-cm scale, their feelings about facial lipoatrophy by answering the following question: What is your satisfaction about the aspect of your face, in relation to the lipoatrophy? Answers were translated into numbers from 0 (total dissatisfaction) to 10 (total satisfaction). The first assessment of VAS score was performed before the first injection. VAS scores were assessed before each following set of injections, at the end of the treatment procedure, and at the end of follow-up. The success rate was defined as the proportion of patients with an increased VAS score as compared with baseline, at the end of the treatment procedure, and at the end of follow-up. The last follow-up visit was the last visit in the study or the visit at which a new set of injections of PLA was judged necessary.
Secondary endpoints included quality of life (QoL) questionnaires, digital photographs (DP), and 3-dimensional photographs (3DP). Patients were assessed at baseline, at the end of the treatment procedure, and at the end of follow-up.
A standardized questionnaire was used for assessing QoL: the Medical Outcome Study Short Form 36-item health survey (MOS SF-36).20,21 The mental and physical component summary scores (MCS and PCS, respectively) (minimum 0, maximum 100) of the MOS SF-36 were analyzed.
Three-dimensional photographs were taken using the DSP 400-TcTI Capture System (3dMD LLC, London, England) and used to measure dermal thickness increase. This device uses simultaneously 2 cameras and allows capturing the texture and geometry of the face. For each photographic session, patients were sitting in the same position, with closed eyes. After 3-dimensional computerized reconstitution of the face, and superimposition of 3DP taken before and after treatment, the thickness of the dermis at the site of injection was calculated. This evaluation was performed for the first 50 treated patients.
Standardized DPs were taken for each patient to visually assess the benefit of the procedure. Two independent assessors were asked to rank in correct order for each patient, DP taken before and after the treatment, and presented in random order.
Statistical Analyses
Data are presented as median (minimum; maximum) for quantitative variables and frequency (percent) for factors. Analysis of efficacy endpoints (VAS, QoL MCS and PCS scores, and 3DP) was performed by comparing values measured at baseline, at the end of therapy, and at the last follow-up visit using paired Wilcoxon tests. Association between patients' baseline characteristics and treatment failure (defined by either a VAS score at the end of treatment lower or equal to baseline, premature discontinuation of the procedure, or loss to follow-up) was tested using Fisher exact tests or Wilcoxon tests. Assessment of agreement between the 2 observers who were asked to rank DP in correct order was performed using kappa statistics. The cumulative incidence of reinjections during follow-up was estimated using Kaplan-Meier product-limit method.
REFERENCES
1. Lo JC, Mulligan K, Tai VW, et al. Buffalo hump in men with HIV infection. Lancet. 1998;351:867-870.
2. Martinez E, Mocroft A, Garcia-Viejo MA, et al. Risk of lipodystrophy in HIV-1-infected patients treated with protease inhibitors: a prospective cohort study. Lancet. 2001;357:592-598.
3. Carr A, Samaras K, Thorisdottir A, et al. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet. 1999;353:2093-2099.
4. Chene G, Angelini E, Cotte L, et al. Role of long-term nucleoside-analogue therapy in lipodystrophy and metabolic disorders in human immunodeficiency virus-infected patients. Clin Infect Dis. 2002;34:649-657.
5. Saves M, Raffi F, Capeau J, et al. Factors related to lipodystrophy and metabolic alterations in patients with human immunodeficiency virus infection receiving highly active antiretroviral therapy. Clin Infect Dis. 2002;34:1396-1405.
6. Joly V, Flandre P, Meiffredy V, et al. Increased risk of lipoatrophy under stavudine in HIV-1-infected patients: results of a substudy from a comparative trial. AIDS. 2002;89:857-862.
7. Kasper TB, Arboleda CH, Halpern M. The impact of patient perceptions of body shape changes and metabolic abnormalities on antiretroviral therapy (abstract WePpB1380). Paper presented at: XIII International AIDS Conference; 9-14 July 2000; Durban, South Africa.
8. Gallego L, Blanco F, Gordillo V, et al. Mental disorders related to lipodystrophy body-shape changes in HIV-positive patients with homosexual risk behaviour (abstract P37). Paper presented at: 2nd International Workshop on Adverse Drug Reactions and Lipodystrophy in HIV; 2000; Toronto, Ontario.
9. Martinez E, Garcia-Viejo MA, Blanch J, et al. Lipodystrophy syndrome in patients with HIV infection. Drug Saf. 2001;24:157-166.
10. Duran S, Savs M, Spire B, et al. Failure to maintain long-term adherence to highly active antiretroviral therapy: the role of lipodystrophy. AIDS. 2001;15:2441-2444.
11. Blanco F, Carr A. Lipodystrophy syndrome: diagnostic, clinic and therapeutic aspects. AIDS Rev. 2001;3:98-105.
12. Currier JS. How to manage metabolic complications of HIV therapy: what to do while we wait for answers. AIDS Read. 2000;10:162-169.
13. Laglenne S. Le new fill. Objectif Peau. 2000;8:58-59.
14. Kronenthal RL. Biodegradable polymers in medicine and surgery. Polym Sci Technol. 1975;8:120-137.
15. Majola A, Vainionpaa S, Vihtonen K, et al. Absorption, biocompatibility, and fixation properties of poly-L-lactic acid in bone tissue: an experimental study in rats. Clin Orthop. 1991;268:260-269.
16. James J, Carruthers A, Carruthers J. HIV-associated facial lipoatrophy. Dermatol Surg. 2002;28:979-986.
17. Amard P, Saint-Marc T, Katz P. The effects of poly-L-lactic acid as therapy for lipoatrophy of the face. Antivir Ther. 2000;5(Suppl):79.
18. Valantin MA, Aubron-Olivier C, Ghosn J, et al. Polylactic acid implants (New-Fill¨) to correct facial lipoatrophy in HIV-infected patients: results of an open-label study. (VEGA). AIDS. 2003;17:2471-2478.
19. Moyle GJ, Lysakova L, Brown S, et al. A randomized open-label study of immediate versus delayed polylactic acid injections for the cosmetic management of facial lipoatrophy in persons with HIV infection. HIV Med. 2004;5:82-87.
20. Wu AW, Rubin HR, Mathews WC, et al. A health status questionnaire using 30 items from the Medical Outcome Study: preliminary validation in persons with early HIV infection. Med Care. 1991;29:786-798.
21. Leplege A, Ecosse E, Pouchot J, et al. Le Questionnaire MOS SF-36: Manuel de L'Utilisateur et Guide d'interprtation des Scores. Edited by Editions Scientifiques Techniques et Mdicales (ESTEM). Paris, France: 2001.
22. Ruiz L, Negredo E, Domingo P, et al. Antiretroviral treatment simplification with nevirapine in protease inhibitor-experienced patients with HIV-associated lipodystrophy: 1-year prospective follow-up of a multicenter, randomized, controlled study. J Acquir Immune Defic Syndr. 2001;27:229-236.
23. Carr A, Workman C, Smith DE, et al. Abacavir substitution for nucleoside analogs in patients with HIV lipoatrophy. JAMA. 2002;288:207-215.
24. Carr A, Workman C, Carey D, et al. No effect of rosiglitazone for treatment of HIV-1 lipoatrophy: randomised, double-blind, placebo-controlled trial. Lancet. 2004;363:429-438.
25. Ritt MJPF, Hillebrand-Haverkort ME, Ten Veen JH. Local treatment of facial lipodystrophy in patients receiving HIV protease inhibitor therapy. Acta Chir Plast. 2001;43:54-56.
26. Levan P, Nguyen TH, Lallemand F, et al. Correction of facial lipoatrophy in HIV-infected patients on highly active antiretroviral therapy by injection of autologous fatty tissue. AIDS. 2002;16:1985-1987.
27. Olenius M. The first clinical study using a new biodegradable implant for the treatment of lips, wrinkles, and folds. Aesthetic Plast Surg. 1998;22:97-101.
28. Rokkanen PU, Bostman O, Hirvensals E, et al. Bioabsorbable fixation in orthopaedic surgery and traumatology. Biomaterials. 2000;21:2607-2613.
29. Bigliardi PL, Izakovic J, Weber JM, et al. Anaphylaxis to the carbohydrate carboxymethylcellulose in parenteral corticosteroid preparations. Dermatology. 2003;207:100-103.
30. Schmid P, Wuthrich B. Peranaesthetic anaphylactoid shock due to mannitol. Allergy. 1992;47:61-62.
31. Orlando G, Guaraldi G, Murri R. Does lipodystrophy affect quality of life? (abstract ThPeB7340). Paper presented at: XIV International AIDS Conference; July 2002; Barcelona.
32. Testa MA, Thompson M, Turner RR, et al. The impact of HIV-associated adipose redistribution syndrome on psychological well-being and quality of life: a cross-sectional survey (abstract ThPeB7341). Paper presented at: XIV International AIDS Conference; July 2002; Barcelona.
|
|
| |
| |
|
|
|