Clinical Infectious Diseases May 1 2005;40:1350-1354
Authors-Yoann Madec,1 Faroudy Boufassa,1 Christine Rouzioux,3 Jean-François Delfraissy,2 and Laurence Meyer,1 for the SEROCO Study Groupa
1Department of Epidemiology, INSERM U569—Paris 11, and 2Department of Medicine, Hôpital de Bicêtre, Le Kremlin-Bicêtre, and 3Department of Virology, Necker Hospital, Paris, France
(See the editorial commentary by Connick et al. below)
"…Our goal was to describe the frequency of seroconverters who had spontaneously achieved consecutive viral load measurements of <400 or <500 copies/mL (depending on the test used) while never having received ART and to describe the duration of this undetectable viremia.
In this study of 426 seroconverters from the SEROCO cohort, which was started in 1988, we found that 36 seroconverters had spontaneously achieved undetectable viremia (>2 consecutive viral load measurements <400 or <500 copies/mL). This phenomenon was not rare: at 5 years after seroconversion, 6.7% of the seroconverters who were followed as part of the cohort still had undetectable viremia. Our definition of undetectable viremia, which required at least 2 consecutive viral load measurements below the detection limit, was strict, because 112 patients (26.3%) had at least 1 viral load measurement below the detection limit. These figures should be kept in mind when assessing the virologic outcome for patients who interrupt HAART initiated during PHI in nonrandomized studies. For example, in one study, no patient had achieved a viral load <500 copies/mL at 1 year after the interruption of HAART [3]; in another study, an undetectable viral load was achieved by 2 of 9 patients for 6 months and by 1 of 9 patients for 18 months after the interruption of HAART [12]…."
Initiation of antiretroviral therapy (ART) during primary HIV infection (PHI) has been suggested to be beneficial, because it can suppress HIV replication and restore the specific anti-HIV CD4+ response [1, 2]. Achieving undetectable viremia during interruption of HAART is often considered to be indicative of the efficacy of short-term treatments initiated during PHI [3, 4]. However, the majority of studies have been open-label studies that did not include a control arm of patients who had received delayed treatment. Data from studies of the natural course of infection that quantify the frequency of undetectable viremia among patients at different times after infection are needed, but such data are sparse. In the Multicenter AIDS Cohort Study, 6.1% of seropositive patients presented with a baseline viral load of <500 copies/mL [5]. Lefrère et al. [6] reported that, among 111 patients with HIV seroconversion (i.e., seroconverters), 15.3% presented with at least 2 viral load measurements (not necessarily consecutive) of <400 copies/mL without having received ART during a mean follow-up period of 8.4 years. In the French SEROCO cohort, 4% of seroconverters spontaneously had an HIV RNA level below the detection limit of 400 copies/mL during the 6—24-month period after infection [7]. The sustainability of this phenomenon during HIV infection has been poorly documented. Goudsmit et al. [8] showed that 14% of 123 seroconverters had achieved viral loads below a threshold of 1000 copies/mL by 1 year after seroconversion; the median time during which viral load remained below the threshold was 2.1 years.
ABSTRACT
Background. The objective of this study was to identify the frequency and characteristics of patients with human immunodeficiency virus (HIV) infection who had spontaneously achieved viremia below the detection limit of either 400 or 500 copies/mL (depending on the test used) and to describe the duration of undetectable viremia without antiretroviral therapy (ART).
Methods. In the French Agence Nationale de Recherches sur le SIDA SEROCO cohort, 426 patients with HIV seroconversion (i.e., seroconverters) enrolled between 1988 and 1995 had serial measurement of HIV RNA levels during follow-up (with a cutoff date of 31 December 2002). Factors that distinguished those patients who had spontaneously achieved undetectable viremia (>2 consecutive viral loads <400 or <500 copies/mL while not receiving ART) were identified by logistic regression. A Cox model was used to estimate the predictive value of factors related to the duration of undetectable viremia.
Results. Undetectable viremia had been spontaneously achieved in 36 of 426 seroconverters. Women (adjusted odds ratio [aOR], 2.44; 95% confidence interval [CI], 1.03—5.80) and subjects with baseline HIV RNA level <3.76 log10 copies/mL (aOR, 0.31; 95% CI, 0.11—0.82), baseline HIV DNA level <2.61 log10 copies/mL (aOR, 0.14; 95% CI, 0.04—0.44), and high baseline CD4+ cell count (aOR, 1.18 for each 100 cells/mm3; 95% CI, 1.03—1.35]) were more likely to have achieved undetectable viremia. The sustainability of this phenomenon (median duration, 11.9 months; range, 4.6—62.8 months) was associated with low baseline HIV DNA and RNA levels.
Conclusions. Achieving undetectable viremia without ART was not rare, because 6.7% of seroconverters still had a viral load of <400 or <500 copies/mL 5 years after seroconversion. These data should be considered when assessing virologic outcome for patients who interrupt highly active ART initiated during primary infection.
AUTHOR DISCUSSION
In this study of 426 seroconverters from the SEROCO cohort, which was started in 1988, we found that 36 seroconverters had spontaneously achieved undetectable viremia (⩾2 consecutive viral load measurements <400 or <500 copies/mL). This phenomenon was not rare: at 5 years after seroconversion, 6.7% of the seroconverters who were followed as part of the cohort still had undetectable viremia. Our definition of undetectable viremia, which required at least 2 consecutive viral load measurements below the detection limit, was strict, because 112 patients (26.3%) had at least 1 viral load measurement below the detection limit. These figures should be kept in mind when assessing the virologic outcome for patients who interrupt HAART initiated during PHI in nonrandomized studies. For example, in one study, no patient had achieved a viral load <500 copies/mL at 1 year after the interruption of HAART [3]; in another study, an undetectable viral load was achieved by 2 of 9 patients for 6 months and by 1 of 9 patients for 18 months after the interruption of HAART [12].
In this study, we considered viral load measurements obtained from 1988 through 2002. Since 1996, improvements and changes in quantification methods have occurred. Nevertheless, the periods of undetectable viremia all started before 1995, when viral load was always measured by use of the Amplicor v1.5 test. Moreover, the end of the periods of undetectable viremia never corresponded to a change in method of measurement.
Predictive factors for undetectable viremia included low baseline HIV RNA and DNA levels and high baseline CD4+ cell count. Women were also more likely to have achieved undetectable viremia, which agrees with previous findings describing lower viral loads in women than in men [13, 14]. The sustainability of undetectable viremia was highly variable: 13 seroconverters still had undetectable viremia 5 years after infection, and 3 seroconverters had undetectable viremia as long as 10 years after infection. The duration of the period of undetectable viremia was associated with low baseline HIV RNA and DNA levels but not with baseline CD4+ cell count.
The decay in CD4+ cell count during and even after the period of undetectable viremia was remarkably slow, compared with the mean decreases in CD4+ cell count described in studies of the natural course of infection [5, 15]. Patients in whom viral load replication is spontaneously controlled over a long period are likely to present with viral and/or immunologic specificities that require further investigation.
RESULTS
Among the 426 seroconverters included in the SEROCO cohort before 1996 who had at least 2 consecutive viral load measurements while never having received ART, 112 (26.3%) had at least 1 viral load measurement below the detection limit. According to our definition (>2 consecutive viral load measurements below the detection limit), 36 seroconverters (8.5%) had achieved undetectable viremia. The time from seroconversion to inclusion in the cohort was a median of 8 months (range, 1— 23 months) and was not significantly different between seroconverters who had achieved undetectable viremia and other seroconverters. CD4+ cell count at inclusion in the cohort was higher among the seroconverters who had achieved undetectable viremia than among the other seroconverters (median CD4+ cell count, 835.5 cells/mm3 vs. 538 cells/mm3, respectively; P < 10-4]). Viral load at inclusion in the cohort was lower among the seroconverters who had achieved undetectable viremia than among the other seroconverters (P < 10-4); 15 patients (41.7%) who had achieved undetectable viremia and 10 patients (2.6%) without a period of undetectable viremia had undetectable viral loads at inclusion. HIV DNA level also was lower among the seroconverters who had achieved undetectable viremia than among the other seroconverters (median HIV DNA level, 2.02 log10 copies/mL vs. 2.90 log10 copies/mL, respectively; P < 10-4).
Factors associated with undetectable viremia are presented in table 1 below. Women were significantly more likely (OR, 4.92; 95% CI, 2.45—9.93) than men to have achieved undetectable viremia. Patients >26 years old (the 33rd percentile of the age distribution) at inclusion were less likely to have a period of undetectable viremia. The CCR5 32 deletion was more frequent among the seroconverters who had achieved undetectable viremia than among the other seroconverters (25.7% vs. 14.9%, respectively), although this association was not statistically significant (P = .10). Type of transmission (blood vs. sexual) was not associated with having achieved undetectable viremia. Seroconverters who had achieved undetectable viremia were not more likely to be infected by a non-B virus subtype than were the other seroconverters (5.7% vs. 8.1%, respectively). A baseline viral load >3.96 log10 copies/mL (33rd percentile) and a baseline HIV DNA level >2.61 log10 copies/mL (33rd percentile) decreased significantly the probability of a period of undetectable viremia. Patients with a high CD4+ cell count at inclusion were more likely to have achieved undetectable viremia. In fact, an increase in CD4+ cell count of 100 cells/mm3 was associated with an OR of 1.33 (95% CI, 1.19—1.48). Women were still more likely to have achieved undetectable viremia than were men, after adjustment for the 3 biological markers, age, and CCR5 genotype. The 3 biological markers remained independently associated with having achieved undetectable viremia in the multivariate analysis.
Table 1. Characteristics of patients in the SEROCO cohort with HIV seroconversion who had achieved undetectable viremia during follow-up, compared with those who had detectable viremia.
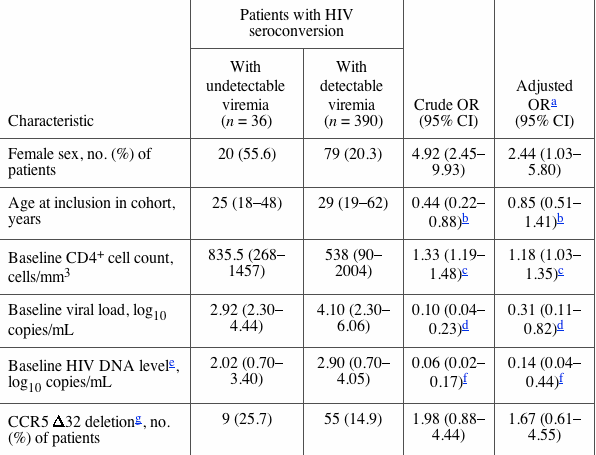
NOTE. Data are median (range), unless otherwise indicated.
a In multivariate logistic regression, adjusted for the 6 variables.
b <26 years (33rd percentile) vs. >26 years.
c For each 100 cells/mm3.
d <3.76 log10 copies/mL (33rd percentile) vs. >3.76 log10 copies/mL.
e Data available for 31 subjects with undetectable viremia and 343 subjects with detectable viremia.
f <2.61 log10 copies/mL (33rd percentile) vs. >2.61 log10 copies/mL.
g Data available for 35 subjects with undetectable viremia and 369 subjects with detectable viremia during follow-up.
In the 36 seroconverters who had achieved undetectable viremia, the delay from seroconversion to undetectable viremia was a median of 21.6 months (range, 1.7—72.0 months). Thirteen patients had undetectable viremia at their first available viral load measurement and a shorter delay between seroconversion and undetectable viremia (median delay, 12.3 months), compared with 23 seroconverters who had a detectable viral load before undetectable viremia was achieved (median delay, 39.0 months). Among these 23 seroconverters, the highest viral load measured before undetectable viremia was achieved was a median of 3.5 log10 copies/mL (range, 2.7—4.5 log10 copies/mL). Of these 23 patients, 2 had an undetectable viral load at baseline followed by 1 measurement of detectable viral load or an interval without measurements of >18 months before undetectable viremia was achieved.
At years 1 and 2 after seroconversion, seroconverters who had achieved undetectable viremia represented 3.5% and 5.5% of the patients, respectively. This proportion was 6.7% at year 5 after seroconversion.
Of the 36 patients who had achieved undetectable viremia, 8 had 2 periods of undetectable viremia. For 4 of these 8 patients, the 2 periods were separated by <12 months during which a single measurement of detectable viral load (not >1200 copies/mL) was observed. For the other 4 patients, viral load measurements were not available for 19, 21, 24, and 48 months; after this period without measurement, a second period of consecutive viral load measurements below the detection limit was observed.
When definition 1 was used, the duration of undetectable viremia was observed to be a median of 11.9 months (range, 4.6—62.8 months); the period of undetectable viremia ended when viral load was above the detection limit (for 29 seroconverters), no viral load measurements were obtained for a period >18 months (for 5 seroconverters), or the follow-up period ended (for 2 seroconverters). When definition 2 was used, the duration of undetectable viremia was a median of 14.5 months (range, 4.6—162.6 months); the period of undetectable viremia ended when viral load was above the detection limit (for 30 seroconverters) or the follow-up period ended (for 6 seroconverters).
Predictive factors for the duration of undetectable viremia by definition 1 were assessed by use of a Cox model. A baseline HIV DNA level >1.96 log10 copies/mL increased the risk of viral load rebound by 3.50-fold (95% CI, 1.36—9.02). A baseline viral load below the detection limit was associated with a longer period of undetectable viremia (relative risk, 2.14; 95% CI, 0.96—4.77). Baseline CD4+ cell count and the interval between seroconversion and the beginning of the period of undetectable viremia were not associated with the duration of the period of undetectable viremia. Similar results were observed when definition 2 for undetectable viremia was used.
At the beginning of the period of undetectable viremia, mean CD4+ cell count was 780 cells/mm3 (95% CI, 693—872 cells/mm3). During the period of undetectable viremia, the mean decrease in CD4+ cell count was 20 cells/mm3 per year. After the period of undetectable viremia and while the patient was still not receiving ART, the mean decrease in CD4+ cell count was 45 cells/mm3 per year.
PATIENTS AND METHODS
Patients.
The Agence Nationale de Recherches sur le SIDA SEROCO cohort study is a multicenter French cohort study that includes 1551 HIV-infected patients recruited since January 1988. Patients are scheduled for a physical examination and laboratory testing every 6 months, at which time serum and cell samples are obtained and stored [9].
Our analysis included patients with a known date of seroconversion who were enrolled within 24 months after infection and who had at least 2 viral load measurements while never having received ART. HIV seroconversion is documented when an interval of <24 months occurs between the last negative and the first positive HIV-antibody test result or between an incomplete Western blot and a complete Western blot. When the above criteria were applied, 426 seroconverters who enrolled before 1996 were identified. The cutoff date for the analysis was 31 December 2002.
Laboratory data.
CD4+ lymphocyte counts were determined at each visit by means of flow cytometry. Before 1996, nearly all HIV RNA levels (99.8%) were determined from frozen serum samples by use of the Amplicor v1.5 HIV Monitor test (detection limit, 400 copies/mL; Roche Diagnostics) [7]. These measurements represented 90% of all the viral loads considered in the analysis. From 1996, HIV RNA was quantitated routinely by use of plasma samples: the Amplicor v1.5 test was used in 75% of cases, the bDNA v2.0 technique (detection limit, 500 copies/mL; Chiron) was used in 24%, and the NASBA QR system (detection limit, 400 copies/mL; Organon Technika) was used in 1%. The Amplicor v1.5 test has been shown to provide slightly higher values for HIV RNA from plasma, compared with from serum, when HIV RNA is detectable [10]. Because only 10% of viral load measurements were obtained after 1996, the change from frozen serum to plasma samples may only have led to an underestimation of the duration of undetectable viremia. The bDNA assay has been shown to lead more easily to the conclusion that HIV RNA is undetectable [11], but this assay was used for only 2.5% of the measurements and only for those obtained after 1996. A comparison between the Amplicor v1.5 and the NASBA assays has led to similar results in terms of undetectability [10].
HIV DNA level in PBMCs was quantified by use of a prototypic assay based on the Amplicor v1.5 monitor test and an HIV DNA internal standard provided by Roche Diagnostics (detection limit, 10 copies/106 PBMCs).
Definition of undetectable viremia.
A patient was considered to have achieved undetectable viremia if at least 2 consecutive viral load measurements were below the detection limit of 400 or 500 copies/mL without the patient having ever received ART and if the time interval between consecutive measurements was <18 months. This time restriction was used to prevent overestimation of the period of undetectable viremia because of missing measurements.
Thus, the period of undetectable viremia consisted of consecutive viral load measurements below the detection limit. The period of undetectable viremia ended when either the viral load was higher than the detection limit, the time interval between 2 consecutive measurements was >18 months, the patient started to receive ART, or the follow-up period ended.
Because a patient could exhibit multiple periods of undetectable viremia, the duration of undetectable viremia was estimated by use of 2 definitions. A strict definition considered the duration of undetectable viremia to be only the first period of undetectable viremia (definition 1), and a broader definition considered the duration of undetectable viremia to be the time from the beginning of the first period to the end of the last period of undetectable viremia (definition 2). This broader definition allowed for temporary increases in viral load or missing measurements.
Statistical methods.
Logistic regression was used to investigate whether patient characteristics, baseline marker levels, a CCR5 32 deletion, or virus subtype (B vs. non-B) could distinguish between seroconverters who had achieved undetectable viremia and other seroconverters. With regard to the patients who had achieved undetectable viremia, a Cox proportional hazards model was used to estimate the association between baseline characteristics and the duration of undetectable viremia. The event of interest was the end of the period of undetectable viremia, as determined by a viral load above the detection limit.
For both models, the log-linear effect of continuous variables was investigated. When this assumption was not valid, a categorical variable was defined for inclusion in the model. A mixed-effect model was used to characterize the progression of CD4 cell count during and after the period of undetectable viremia. SAS software, version 8.2 (SAS Institute), was used for the statistical analyses.
References
1. Harrigan PR, Whaley M, Montaner JSG. Rate of HIV-1 RNA rebound upon stopping antiretroviral therapy. AIDS 1999; 13:F59—62. First citation in article | PubMed | CrossRef
2. Garcia F, Plana M, Vidal C, et al. Dynamics of viral load rebound and immunological changes after stopping effective antiretroviral therapy. AIDS 1999; 13:F79—86. First citation in article | PubMed
3. Markowitz M, Jin X, Hurley A, et al. Discontinuation of antiretroviral therapy commenced early during the course of human immunodeficiency virus type 1 infection, with or without adjunctive vaccination. J Infect Dis 2002; 186:634—43. First citation in article | Full Text | PubMed
4. Rosenberg ES, Altfeld M, Poon SH, et al. Immune control of HIV-1 after early treatment after acute infection. Nature 2000; 407:523—6. First citation in article | PubMed | CrossRef
5. Mellors JW, Rinaldo CR, Gupta P, White RM, Todd JA, Kingsley LA. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 1996; 272:1167—70. First citation in article | PubMed
6. Lefrère J-J, Mariotti M, Morand-Joubert L, Thauvin M, Roudot-Thoraval F. Plasma human immunodeficiency virus RNA below 40 copies/mL is rare in untreated persons even in the first years of infection. J Infect Dis 1999; 180:526—9. First citation in article | Full Text | PubMed
7. Hubert JB, Burgard M, Dussaix E, et al. Natural history of serum HIV-1 RNA levels in 330 patients with a known date of infection. The SEROCO Study Group. AIDS 2000; 14:123—31. First citation in article | PubMed | CrossRef
8. Goudsmit J, Bogaards JA, Jurriaans S, et al. Naturally HIV-1 seroconverters with lowest viral load have best prognosis, but in time lose control of viraemia. AIDS 2002; 16:791—3. First citation in article | PubMed | CrossRef
9. Carre N, Boufassa F, Hubert J-B, et al. Predictive value of viral load and other markers for progression to clinical AIDS after CD4+ cell count falls below 200/mL. Int J Epidemiol 1998; 27:897—903. First citation in article | PubMed | CrossRef
10. Griffith BP, Rigsby MO, Garner RB, Gordon MM, Chacko TM. Comparison of the Amplicor HIV-1 monitor test and the nucleic acid—based amplification assay for quantification of human immunodeficiency virus RNA in plasma, serum and plasma subjected to freeze-thaw cycles. J Clin Microbiol 1997; 35:3288—91. First citation in article | PubMed
11. Coste J, Montes B, Reynes J, et al. Comparative evaluation of three assays for the quantification of human immunodeficiency virus type 1 RNA in plasma. J Med Virol 1996; 50:293—302. First citation in article | PubMed | CrossRef
12. Girard PM, Schneider V, Dehée A, et al. Treatment interruption after one year of triple nucleoside analogue therapy for primary HIV infection. AIDS 2001; 15:275—7. First citation in article | PubMed | CrossRef
13. Farzadegan H, Hoover DR, Astemborski J, et al. Sex differences in HIV-1 viral load and progression to AIDS. Lancet 1998; 352:1510—4. First citation in article | PubMed | CrossRef
14. Sterling TR, Lyles CM, Vlahov D, Astemborski J, Margolick JB, Quinn TC. Sex differences in longitudinal human immunodeficiency virus type 1 RNA levels among seroconverters. J Infect Dis 1999; 180:666—72. First citation in article | Full Text | PubMed
15. Lyles RH, Munoz A, Yamashita TE, et al. Natural history of human immunodeficiency virus type 1 viremia after seroconversion and proximal to AIDS in a large cohort of homosexual men. Multicenter AIDS Cohort Study. J Infect Dis 2000; 181:872—80.
EDITORIAL COMMENTARY
Challenges in the Study of Patients with HIV Type 1 Seroconversion
Elizabeth Connick,1 Samantha MaWhinney,2 Cara C. Wilson,1 and Thomas B. Campbell1
1Division of Infectious Diseases and 2Department of Preventive Medicine and Biometrics, University of Colorado at Denver and Health Sciences Center, Denver
In this issue of Clinical Infectious Diseases, Madec et al. [1] report that spontaneous and sustained clearance of HIV type 1 (henceforth, HIV) viremia (defined as repeated HIV RNA levels in plasma of <500 copies/mL) is not uncommon in persons recently infected with HIV. Undetectable viremia occurred in 4% and 6% of 426 individuals in the French SEROCO cohort within 1 and 2 years after infection, respectively, and was observed in of these persons at 5 years after infection. Low-level viremia is recognized to be correlated with long-term nonprogressive HIV infection [2]. Thus, it is an important clinical end point for studies of interventions during acute-phase and early-phase HIV infection. Nevertheless, use of low-level viremia to interpret the impact of treatments in nonrandomized studies of patients with HIV seroconversion (i.e., HIV seroconverters) is problematic.
Discrepancies in reported frequencies of undetectable viremia among HIV seroconverters highlight the difficulty of using such studies to interpret the results of treatment trials. Among an Amsterdam cohort of 123 HIV seroconverters, plasma HIV RNA level was <1000 copies/mL in 14% of untreated HIV seroconverters 1 year after seroconversion, but only 2% had virus loads <1000 copies/mL 5 years after seroconversion [3]. These data suggest a substantially higher incidence of baseline low-level viremia among HIV seroconverters than that reported by Madec et al. [1] but a lower incidence of sustained low-level viremia 5 years after seroconversion. Long-term rates of undetectable viremia have also varied in studies of HAART-treated HIV seroconverters. Of 14 HIV seroconverters who were selected on the basis of intact HIV—specific lymphoproliferative responses after having received HAART, 4 (29%) had episodes of viremia of <500 copies/mL during ⩾1 structured treatment interruption (STI), and 1 (7%) had sustained plasma HIV RNA concentrations below that level a median of 5.3 years after infection [4], which is similar to the results reported by Madec et al. On the other hand, in 2 other small studies of HAART-treated HIV seroconverters who had received early antiretroviral therapy and, in some cases, HIV vaccination, sustained viremia of <500 copies/mL within the first 1.5 years of discontinuation of antiretroviral treatment did not occur [5, 6].
Discrepant prevalences of undetectable viremia during follow-up are likely to be, at least in part, due to differences in the study participants' characteristics. Although as many as 90% of HIV seroconverters experience some sort of clinical illness associated with primary HIV infection [7], most individuals are unaware that their symptoms could indicate HIV infection, and many do not seek medical treatment. Of those individuals who seek medical care for symptoms of primary infection, the correct diagnosis is often overlooked during medical evaluation [7—9]. Severe and prolonged symptoms [10—12], as well as a history of having sought medical attention for symptoms of primary infection [13], have been correlated with higher virus loads and more-rapid progression of disease. Thus, studies that rely heavily on the identification of HIV seroconverters by clinical symptoms are likely to be biased toward individuals with aggressive disease. Such bias is substantially less likely in studies in which HIV seroconverters were identified retrospectively from archived serial specimens obtained from men who have sex with men, such as in the Multicenter AIDS Cohort Study (MACS) [14] or the Amsterdam cohort study [3]. Nevertheless, even these studies contain inherent sources of bias. The study by Madec et al. [1], as well as previous studies [15], have shown that women, compared with men, have significantly lower plasma HIV RNA concentrations up to 5 years after seroconversion, suggesting that the MACS and the Amsterdam cohort study overestimated virus loads in mixed populations of men and women. Individuals who are experiencing symptoms of seroconversion may not appear for required cohort-study visits, which may lead to discontinued participation in the study. Indeed, those rare individuals who develop opportunistic infections at the time of seroconversion and those who die from AIDS within the first year of seroconversion also would be less likely to be included in these cohorts, suggesting additional bias toward subjects with slower disease progression. Although behavioral correlates of disease progression have not yet been identified, it is not implausible that behavior that may predispose patients to rapid disease progression, such as multiple sex partners leading to superinfection [16], could be correlated with diminished participation in research studies.
Once HIV seroconverters are identified, there are additional potential sources of selection bias related to their enrollment in studies. Individuals who present with opportunistic infections or encephalitis during seroconversion and, consequently, have a poor prognosis may be excluded from participation in clinical trials because of those symptoms. Assessment of the potential effect of these issues on study findings requires that investigators report the reasons for exclusion of subjects or for discontinuation of participation, as well as provide a comparison of included and excluded subjects. Retention of subjects in a research study and the regularity of follow-up for study visits also could be affected by the rapidity and severity of disease progression. Decreases in CD4+ T cell counts and increases in virus loads may precipitate treatments that result in removal of subjects from a study, thereby creating a bias in the data set toward subjects with less-aggressive disease. Indeed, it was reported previously that 41% of HIV seroconverters enrolled in the SEROCO cohort before February 1996 had received antiretroviral therapy before they developed AIDS [17]. In the study by Madec et al. [1], 13 individuals in the SEROCO cohort were observed to have undetectable viremia at year 5, and individuals who received antiretroviral therapy were excluded from the analysis. Consideration of this observation in combination with the reported rate of undetectable viremia of 6.7% at year 5 suggests that less than half of the initial 426 subjects remained eligible. Thus, in this study, the reported rate of undetectable virus load at year 5 may have overestimated the true rate among HIV seroconverters.
The interval between study visits also may introduce bias into results such as the identification of subjects who spontaneously achieve and maintain undetectable viremia. Virus load measurements taken closer together have more correlation than those taken farther apart [18]. Therefore, subjects with 1 undetectable virus load are more likely to demonstrate undetectable viremia if the follow-up virus load measurement is taken sooner rather than later. Subjects with only 2 measurements also have a reduced chance of success, compared with subjects with multiple measurements [19]. Average time between visits, follow-up time, and recruitment year should be considered as potential confounders in logistic regression analyses. Long intervals between study visits can lead to an overestimation of the duration of undetectable viremia [20]. Between visits, patients may have detectable virus loads that are either missed or not identified until the following visit. Statistical methods that fail to compensate for intervals in data collection can lead to misleading results [21, 22].
The multiple potential sources of bias involved in recruitment and retention of HIV seroconverters in studies indicate that it is perilous to use historical data as a control in the interpretation of clinical outcomes among HIV seroconverters. Unfortunately, data from an uncontrolled study of 8 HIV seroconverters [23] were widely interpreted to show that early treatment of HIV infection with HAART followed by STI led to a lower virus set point, implying significant clinical benefit. This interpretation fostered a widespread perception that withholding therapy from HIV seroconverters, particularly those with acute HIV infection, was unethical. As a consequence, few randomized clinical trials of treatment of HIV seroconverters have been performed [24]. The results of longer follow-up now suggest that the clinical benefits initially observed were not sustained over the long term in HIV seroconverters with acute-phase infection who received early treatment followed by STI [4]–although, again, these studies were not controlled. The lack of randomized controlled clinical trials of interventions among patients with acute and recent HIV infection has been a great disservice to HIV-infected patients and has left clinicians caring for such patients without clear treatment guidelines. It is imperative that clinicians and researchers realize the importance of randomized controlled studies in validating or refuting anecdotal and uncontrolled observations of the treatment received by HIV seroconverters. Although studies of HIV seroconverters pose multiple logistical, statistical, and clinical challenges, the proper design and performance of these studies are critical to gain better insight into HIV immunopathogenesis and optimal treatment strategies.
References
1. Madec Y, Boufassa F, Rouzioux C, Delfraissy J-F, Meyer L. Undetectable viremia without antiretroviral therapy in patients with HIV seroconversion: an uncommon phenomenon? The SEROCO Study Group. Clin Infect Dis 2005; 40:1350—4 (in this issue). First citation in article
2. Mellors JW, Rinaldo CR Jr, Gupta P, White RM, Todd JA, Kingsley LA. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 1996; 272:1167—70. First citation in article | PubMed
3. Goudsmit J, Bogaards JA, Jurriaans S, et al. Naturally HIV-1 seroconverters with lowest viral load have best prognosis, but in time lose control of viraemia. AIDS 2002; 16:791—3. First citation in article | PubMed | CrossRef
4. Kaufmann DE, Lichterfeld M, Altfeld M, et al. Limited durability of viral control following treated acute HIV infection. PLoS Med 2004; 1:137—48. First citation in article | PubMed | CrossRef
5. Girard PM, Schneider V, Dehée A, et al. Treatment interruption after one year of triple nucleoside analogue therapy for primary HIV infection. AIDS 2001; 15:275—7. First citation in article | PubMed | CrossRef
6. Markowitz M, Jin X, Hurley A, et al. Discontinuation of antiretroviral therapy commenced early during the course of human immunodeficiency virus type 1 infection, with or without adjunctive vaccination. J Infect Dis 2002; 186:634—43. First citation in article | Full Text | PubMed
7. Schacker T, Collier AC, Hughes J, Shea T, Corey L. Clinical and epidemiologic features of primary HIV infection. Ann Intern Med 1996; 125:257—64. First citation in article | PubMed
8. Clark SJ, Kelen GD, Henrard DR, et al. Unsuspected primary human immunodeficiency virus type 1 infection in seronegative emergency department patients. J Infect Dis 1994; 170:194—7. First citation in article | PubMed
9. Rosenberg ES, Caliendo AM, Walker BD. Acute HIV infection among patients tested for mononucleosis. N Engl J Med 1999; 340:969. First citation in article | PubMed | CrossRef
10. Henrard DR, Phillips JF, Muenz LR, et al. Natural history of HIV-1 cell-free viremia. JAMA 1995; 274:554—8. First citation in article | PubMed | CrossRef
11. Dorrucci M, Rezza G, Vlahov D, et al. Clinical characteristics and prognostic value of acute retroviral syndrome among injecting drug users. Italian Seroconversion Study. AIDS 1995; 9:597—604. First citation in article | PubMed
12. Pedersen C, Lindhardt BO, Jensen BL, et al. Clinical course of primary HIV infection: consequences for subsequent course of infection. BMJ 1989; 299:154—7. First citation in article | PubMed
13. Schacker TW, Hughes JP, Shea T, Coombs RW, Corey L. Biological and virologic characteristics of primary HIV infection. Ann Intern Med 1998; 128:613—20. First citation in article | PubMed
14. Lyles RH, Munoz A, Yamashita TE, et al. Natural history of human immunodeficiency virus type 1 viremia after seroconversion and proximal to AIDS in a large cohort of homosexual men. Multicenter AIDS Cohort Study. J Infect Dis 2000; 181:872—80. First citation in article | Full Text | PubMed
15. Sterling TR, Lyles CM, Vlahov D, Astemborski J, Margolick JB, Quinn TC. Sex differences in longitudinal human immunodeficiency virus type 1 RNA levels among seroconverters. J Infect Dis 1999; 180:666—72. First citation in article | Full Text | PubMed
16. Grobler J, Gray CM, Rademeyer C, et al. Incidence of HIV-1 dual infection and its association with increased viral load set point in a cohort of HIV-1 subtype C—infected female sex workers. J Infect Dis 2004; 190:1355—9. First citation in article | Full Text | PubMed
17. Hubert JB, Burgard M, Dussaix E, et al. Natural history of serum HIV-1 RNA levels in 330 patients with a known date of infection. The SEROCO Study Group. AIDS 2000; 14:123—31. First citation in article | PubMed | CrossRef
18. Brown ER, MaWhinney S, Jones RH, Kafadar K, Young B. Improving the fit of bivariate smoothing splines when estimating longitudinal immunological and virological markers in HIV patients with individual antiretroviral treatment strategies. Stat Med 2001; 20:2489—504. First citation in article | PubMed | CrossRef
19. Rothman K. Modern epidemiology. Boston and Toronto: Little, Brown, and Company, 1986. First citation in article
20. Turnbull BW. The empirical distribution function with arbitrarily grouped, censored and truncated data. J R Stat Soc [Ser B] 1976; 38:290—5. First citation in article
21. Betensky RA, Finkelstein DM. Testing for dependence between failure time and visit compliance with interval-censored data. Biometrics 2002; 58:58—63. First citation in article | PubMed | CrossRef
22. Lindsey JC, Ryan LM. Tutorial in biostatistics methods for interval-censored data. Stat Med 1998; 17:219—38. First citation in article | PubMed | CrossRef
23. Rosenberg ES, Altfeld M, Poon SH, et al. Immune control of HIV-1 after early treatment of acute infection. Nature 2000; 407:523—6. First citation in article | PubMed | CrossRef
24. Smith DE, Walker BD, Cooper DA, Rosenberg ES, Kaldor JM. Is antiretroviral treatment of primary HIV infection clinically justified on the basis of current evidence? AIDS 2004; 18:709—18.