| |
"....Abnormal kidney function may not be recognized among patients who have lower relative muscle mass (e.g., women, older patients, and patients with lower lean body weights due to cachexia or liver disease) using serum creatinine level alone...."
".....There is a great need for well-designed, prospective studies evaluating the natural history of kidney diseases in HIV-infected patients in both the adult and the pediatric populations...."
Clinical Infectious Diseases, June 1, 2005;40:1559-1585
Samir K. Gupta,1 Joseph A. Eustace,3 Jonathan A. Winston,4 Ivy I. Boydstun,5 Tejinder S. Ahuja,7 Rudolph A. Rodriguez,8 Karen T. Tashima,10 Michelle Roland,9 Nora Franceschini,11 Frank J. Palella,13 Jeffrey L. Lennox,14 Paul E. Klotman,4 Sharon A. Nachman,6 Stephen D. Hall,2 and Lynda A. Szczech12
Divisions of 1Infectious Diseases and 2Clinical Pharmacology, Department of Medicine, Indiana University School of Medicine, Indianapolis; 3Division of Nephrology, Johns Hopkins University, School of Medicine and Department of Epidemiology, Bloomberg School of Public Health, Baltimore, Maryland; 4Division of Nephrology, Department of Medicine, Mount Sinai School of Medicine, New York, and 5Division of Nephrology and Hypertension and 6Division of Infectious Diseases, Department of Pediatrics, State University of New York, Stony Brook; 7Division of Nephrology, Department of Medicine, University of Texas Medical Branch, Galveston; 8Division of Nephrology, Department of Medicine, San Francisco General Hospital and 9Positive Health Program at San Francisco General Hospital and the UCSF AIDS Research Institute, Department of Medicine, University of California at San Francisco; 10Division of Infectious Diseases, Department of Medicine, The Miriam Hospital, Brown Medical School, Providence, Rhode Island; 11Division of Nephrology and Hypertension, Department of Medicine, University of North Carolina at Chapel Hill, and 12Duke Clinical Research Institute and the Division of Nephrology, Department of Medicine, Duke University Medical Center, Durham, North Carolina; 13Division of Infectious Diseases, Department of Medicine, Northwestern University Feinberg School of Medicine, Chicago, Illinois; and 14Grady Infectious Disease Program, Division of Infectious Diseases, Department of Medicine, Emory University, Atlanta, Georgia
".....As HIVAN predominantly affects black persons, it is not surprising that most HIV-infected ESRD patients (87.8%) are African American.... decreased CD4+ cell count and a family history of renal disease are risk factors for the development of HIVAN..."....diabetes, hypertension, hepatitis C coinfection, patients with HIV RNA levels >4000 copies/ml.
".....Hypertension is both a cause of CKD in the general population and a proven risk factor for faster progression towards dialysis. The prevalence of hypertension in unselected HIV-infected patients is 12%-21%..." see discussion below in Management section.
EXECUTIVE SUMMARY
Several lines of evidence point to kidney disease as an important complication of human immunodeficiency virus (HIV) infection. Kidney function is abnormal in up to 30% of HIV-infected patients, AIDS-related kidney disease has become a relatively common cause of end-stage renal disease (ESRD) requiring dialysis, and kidney disease may be associated with progression to AIDS and death [1-4]. Because HIV caregivers commonly manage all aspects of treatment for their patients, these clinicians are in the unique and important position to identify those patients at risk for renal disease and implement potentially preventative and therapeutic strategies. Consequently, an understanding of the causes, epidemiology, screening methods, and therapeutic strategies associated with chronic kidney disease (CKD) in the HIV-infected patient is required.
However, clinical research in this emerging area has not yet matured to the point at which it can provide clear evidence on how best to treat these patients. Therefore, assembled clinical experts in this field have reviewed the available literature and have offered the following recommendations, many of which are extrapolated from research and clinical guidelines [5] involving the general population with kidney disease. These guidelines address the clinical issues involved in both adults and children with HIV-related renal diseases and are written for those providing inpatient and outpatient care for these patients and for the patients themselves. Although the authors feel that these recommendations should generally apply to all HIV-infected patients, it is understood that providers need to tailor these guidelines around the needs and circumstances of the individual patient.
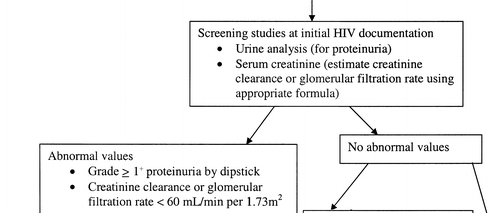
We recommend that all patients at time of HIV diagnosis be assessed for existing kidney disease with a screening urine analysis for proteinuria and a calculated estimate of renal function (creatinine clearance or glomerular filtration rate [GFR]) (C-III); a renal function estimate also allows the caregiver to properly prescribe those antiretrovirals and other commonly used medications that require renal adjustment.
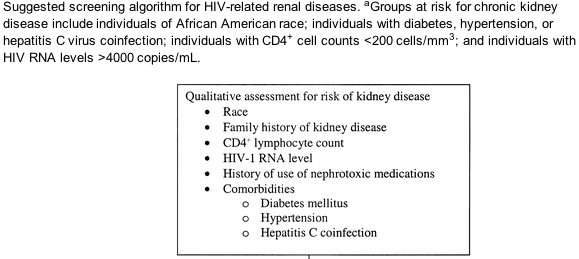
If there is no evidence of proteinuria at initial evaluation, patients at high risk for the development of proteinuric renal disease (e.g., African American persons, those with CD4+ cell counts <200 cells/mL or HIV RNA levels >4000 copies/mL, or those with diabetes mellitus, hypertension, or hepatitis C virus coinfection) should undergo annual screening (B-II).
Patients with proteinuria of grade >1+ by dipstick analysis or reduced renal function (GFR, <60 mL/min per 1.73 m2) should be referred to a nephrologist and undergo additional evaluation, including quantification of proteinuria, renal ultrasound, and potentially renal biopsy (B-II).
Therapy for HIV-associated renal diseases should be individualized to the patient's clinical circumstances and to the underlying renal histology findings. Blood pressure should be controlled, with the initial preferential use of angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers for those patients with proteinuria (B-II); however, calcium channel blockers should be avoided in treating patients receiving protease inhibitors (D-II).
Patients with HIV-associated nephropathy (HIVAN) should be treated with HAART at diagnosis (B-II). HAART should not be withheld from patients simply because of the severity of their renal dysfunction (B-III). Addition of ACE inhibitors or angiotensin receptor blockers (ARBs) should be considered in treating both adult and pediatric patients with HIVAN if HAART alone does not result in improvement of renal function (B-II).
Prednisone should also be considered in treating adult patients with refractory HIVAN (B-II), although steroids are not recommended for children with HIVAN (D-II).
Preliminary data suggest that renal transplantation may be a viable treatment option for patients with ESRD and should be considered if provided in a supervised clinical trial or at centers with adequate experience in this area (C-III).
Dialysis and the placement of arteriovenous fistulae should not be withheld for patients solely because of HIV infection (A-II).
Among those patients at higher risk (see "Renal Effects of Commonly Used Medications in HIV Care" below in this article), biannual monitoring for renal function and urinary abnormalities is warranted for those receiving indinavir (B-II) or tenofovir (B-III).
HIV-infected patients requiring hemodialysis should have antibody to hepatitis B surface antigen (anti-HBs) titers checked after receiving a standard primary series of 3 hepatitis B vaccinations, and they should receive a fourth injection if these levels are <10 IU/L (B-II).
INTRODUCTION
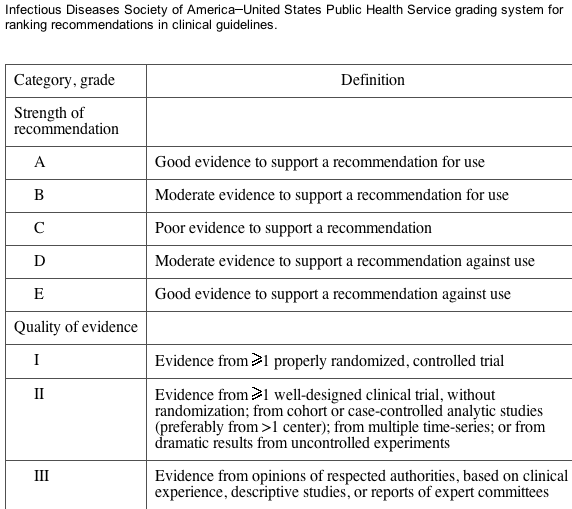
We first provide general reviews regarding the evaluation of renal disease and the epidemiology of CKD in the HIV-infected population; these are followed by specific recommendations for the management of cases in this population. The evidence for these guidelines was collected using MEDLINE searches of the relevant literature and reviews of pertinent abstracts (all in the English language) presented at both major infectious diseases and nephrology society meetings from January 2000 through February 2004. The evidence was graded using the Infectious Diseases Society of America-United States Public Health Service system for rating recommendations in clinical guidelines (table 1). These guidelines will be updated periodically as evidence from the ongoing research in CKD epidemiology, antiretroviral pharmacokinetics, treatment strategies for progressive nephropathies, and renal transplantation in the HIV-infected population accumulate.
Table 1.
Acute renal failure. An important first step in evaluating kidney disease is to distinguish acute renal failure (ARF) from CKD. ARF is a clinical syndrome defined as an abrupt decrease in GFR over days to weeks. For study toxicity-grading purposes, the Adult AIDS Clinical Trials Group has defined ARF as an increase in serum creatinine level to values >1.5 mg/dL (or >1.3 times the upper limit of normal at the respective clinical laboratory) that returns to baseline values within 3 months. Among outpatients, ARF is commonly caused by drug-specific renal toxicities (see "Renal Effects of Commonly Used Medications in HIV Care") and prerenal states associated with dehydration. Recommendations for diagnosing, preventing, and treating ARF are beyond the focus of the current recommendations.
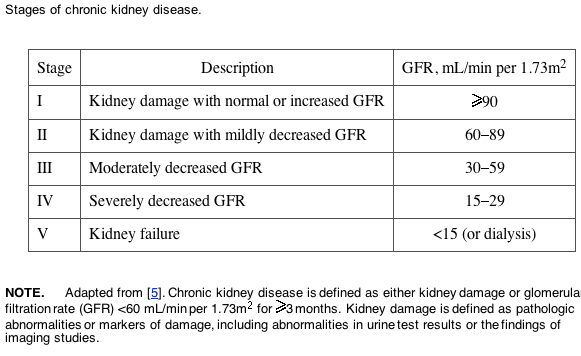
CKD. A standardized nomenclature for chronic diseases of the kidney has only recently been established. The reader will find several terms in the medical literature that are used interchangeably and often lack precision, such as "chronic renal failure" or "chronic renal insufficiency." The National Kidney Foundation has endorsed the term "chronic kidney disease (CKD)," defined as evidence of kidney damage that persists for >3 months [5]. As shown in table 2, the severity of CKD is graded according to renal function, on the basis of estimates of either the creatinine clearance (calculated using the Cockcroft-Gault equation) [6] or the GFR (calculated using the modification of diet in renal disease [MDRD] equation) [7]. Note that ESRD is defined as stage V CKD.

Abnormal kidney function may not be recognized among patients who have lower relative muscle mass (e.g., women, older patients, and patients with lower lean body weights due to cachexia or liver disease) using serum creatinine level alone. Equations adjusting for surrogates of muscle mass (e.g., age, weight, race, and sex), therefore, provide a more sensitive estimation of true renal function. The full MDRD equation also adjusts for serum albumin level. A simplified MDRD equation [8] that does not rely on albumin levels or weight is also highly accurate. These equations have not been validated for the HIV-infected population; therefore, there is no absolutely preferred equation to use consistently among patients with HIV infection. For CKD staging purposes, the simplified MDRD equation is, in general, preferred. Because studies of medications in renal failure have traditionally used the Cockcroft-Gault equation, it would be appropriate to use this estimating formula in deciding on dosage. Versions of these equations are available in electronic format for use online at the Web site of the Kidney Disease Outcomes Quality Initiative of the National Kidney Foundation (K/DOQI) [9] and are presented below.
The CKD staging system also uses other evidence of kidney disease (such as albuminuria, proteinuria, or abnormal findings on imaging of the kidneys) to assist in identifying early renal dysfunction when serum creatinine and/or GFR are normal. Screening for early stages of CKD, therefore, requires measurement of urinary albumin-to-creatinine or protein-to-creatinine ratios. These "spot" quantitative urine measurements of abnormal glomerular function are accurate, correlate with 24-h urine measurements, and avoid the inconvenience and difficulty in collection of timed urine specimens in clinical practice [10]. Patients with stage III and stage IV CKD have more-severe reduction in GFR. These patients are at high risk for developing ESRD (stage V CKD) and death and should, therefore, be carefully evaluated to determine the etiology and severity of their disease.
The benefits of screening for stage I-II CKD have been unequivocally demonstrated in diabetic kidney disease, in which identifying and treating patients with microalbuminuria (a urinary albumin-to-creatinine ratio >30 mg/g), macroalbuminuria (an albumin-to-creatinine ratio of ⩾300 mg/g), or overt proteinuria (a protein-to-creatinine ratio of >300 mg/g) can slow or prevent the progression of kidney disease [11, 12]. Screening for albuminuria and proteinuria has also become an important tool for identifying those at increased risk for atherosclerotic vascular disease [13].
EPIDEMIOLOGY
Spectrum of HIV-related renal diseases. Up to 60% of all renal biopsies performed for patients with CKD reveal characteristic histological findings designated as HIVAN, which is a collapsing form of focal glomerulosclerosis (FSGS) with tubulointersitial injury, most often presenting as the nephrotic syndrome. Increasing evidence implicates direct intrarenal HIV infection and expression of its genes in the pathogenesis of HIVAN [14-17]. In addition to HIVAN, specific renal histological conditions encountered among patients with HIV infection include membranous nephropathy resulting from coinfection with either hepatitis B or C or syphilis; membranoproliferative glomerulonephritis associated with hepatitis C virus coinfection and mixed cryoglobulinemia [18, 19]; diabetic and hypertensive nephropathies [20]; and immune complex glomerulonephritis, in which IgA is directed against HIV antigens [21]. There is no evidence in the literature that suggests a definitive way other than renal biopsy to distinguish patients with HIVAN from patients with diseases other than HIVAN. However, patients with glomerular diseases other than HIVAN are less likely to be black, more likely to have hepatitis B virus infection, less likely to have the diagnosis of hypertension, and have a greater mean CD4+ lymphocyte count [19].
Incidence, prevalence, and risk factors of CKD. In 2000, 1.5% (range, 0.3%-3.4%) and 0.4% (range, 0%-1.0%) of dialysis patients were reported to have HIV infection and AIDS, respectively [22]. From 1985 through 2000, the percentage of centers that reported providing dialysis for patients with HIV infection increased from 11% to 37%. Because dialysis patients in the United States do not necessarily undergo routine screening for HIV infection, true incidence and prevalence estimates are probably higher than those reported by either the Centers for Disease Control and Prevention (CDC) or the United States Rendal Data System (USRDS).
Incidence and prevalence rates of HIV-infected patients with ESRD reported by various studies utilizing the USRDS database have demonstrated similar results [23, 24]. The incident number of patients with HIVAN undergoing dialysis each year increased through 1995, when 939 patients with HIVAN initiated dialysis. Since then, the number has remained stable. This salutary effect on the incidence of ESRD secondary to HIVAN likely reflects changes in the natural history of HIV disease consequent to the use of HAART. During the years 1995-1999, the incidence of ESRD secondary to HIVAN for African American men aged 25-44 years decreased from 8.5% to 6.8%. However, the number of prevalent cases increased from 1346 (0.4%) to 3058 (0.8%), and the 1-year survival rate improved from 52% to 69% [25]. As HIVAN predominantly affects black persons, it is not surprising that most HIV-infected ESRD patients (87.8%) are African American [26]. HIVAN had a stronger association with black race than with any other cause of renal failure except sickle cell anemia. The prevalence of HIVAN among HIV-infected black patients has been reported to be 3.5% in a cohort screened for proteinuria in a primary care setting [27].
In addition to African American race [28-35], decreased CD4+ cell count [31, 36] and a family history of renal disease [37] are risk factors for the development of HIVAN. Older reports suggest that injection drug use [28-32, 38, 39] may also be a risk factor, but this likely represents confounding with the other epidemiologic factors (e.g., socioeconomic status, race, and hepatitis C virus coinfection) from the early stages of the HIV epidemic. Although male sex has also been reported as a risk factor [28, 35], the incidence of HIVAN in black women is increasing proportionately to the escalating rate of HIV infection in this group [26, 40, 41], strongly suggesting that HIVAN is not sex-specific. A large series of patients with HIVAN (n = 102) confirmed that black race is a major risk factor for the presence of HIVAN but suggests that sex, risk factors for HIV acquisition, and CD4+ lymphocyte count at the time of diagnosis are not as significant as previously described [42]. The currently available epidemiological data may be limited by potential biases introduced through selection of patients following referral to a nephrologist or by the performance of either biopsy or autopsy. Patients identified in such a manner may have a disproportionately greater severity of renal disease or a more aggressive course.
Specific factors that have been associated with decreases in renal function among HAART-treated women include higher baseline HIV load (>4000 copies/mL), lower baseline CD4+ cell count (<200 cells/mL), a diagnosis of diabetes, and hypertension [1]. Recent reports have also suggested that the baseline presence of proteinuria, with or without concomitant elevations in the serum creatinine level, is a sensitive prognosticator of the eventual development of CKD [2, 3]. The prevalence of grade 1+ proteinuria by dipstick analysis of urine, a marker for glomerular disease, is 〜30% [1, 2, 43]. In the CDC-sponsored HIV Epidemiology Research Study Group (HERS), 2+ proteinuria (found in 7.2% of subjects) and/or elevated serum creatinine levels (2.4%) were frequently noted on initial visit among urban, HIV-infected women; these conditions subsequently developed in an additional 14% and 4%, respectively, over a median of 21 months [3].
Survival of the HIV-infected patient with CKD. Early studies reported that persons with newly diagnosed AIDS and ESRD survived a mean of 1-3 months after initiating hemodialysis [29, 44]. Such studies predominantly reflected the clinical course of patients with advanced HIV disease who often had other opportunistic diseases. As screening for HIV became more routine and resulted in earlier detection, several groups reported improved survival among HIV-infected dialysis patients beginning in the early 1990s [22, 45-49]. Although HIV-infected patients undergoing dialysis in the HAART era have experienced improvements in survival [45], such benefits may still be attenuated when compared with the general HAART-treated HIV-infected population [50].
Recent data from the USRDS has demonstrated that the mortality rates for patients with AIDS-related nephropathy have improved, compared with mortality rates in pre-HAART era reports, and are now approaching the mortality rates in the general ESRD population. The first-year survival rate for HIV-infected patients with ESRD (who may or may not be receiving HAART) has increased to 74%; the annual death rates for HIV-infected and HIV-uninfected patients receiving dialysis are now 240.2 deaths per 1000 patient-years and 236.4 deaths per 1000 patient-years, respectively [24]. Several reports have demonstrated the maintenance of stable renal function among HAART recipients, compared with those not receiving HAART [51, 52], even in patients with advanced HIV disease [53].
In the aforementioned CDC-sponsored HERS study, the presence of proteinuria and/or elevated serum creatinine levels was positively associated with an increased risk of death (adjusted relative hazard, 2.5), as well as with having renal causes of death recorded on death certificates (26% of total deaths) [3]. Likewise, renal laboratory abnormalities were associated with higher hospitalization rates (adjusted relative risk, 1.5) [54]. A recent report from the Women's Interagency HIV Study Cohort further elucidated that proteinuria and elevated serum creatinine levels have been associated with greater mortality both before and after initiation of HAART [4]. In this study, although proteinuria was positively associated with a risk of AIDS-defining illness in the pre-HAART era, elevated serum creatinine levels remained a predictor of AIDS-defining illness in the HAART era [4]. Moreover, other recent data suggest that, in an era when overall AIDS-related mortality and rates of traditional AIDS-related opportunistic infections remain very low among HAART-treated patients, the presence of severe renal abnormalities and dysfunction are significantly associated with mortality [55, 56].
SCREENING AND INITIAL EVALUATIONS
Recommendation 1. All patients at the time of HIV diagnosis should be assessed for existing kidney disease with a screening urine analysis for proteinuria and a calculated estimate of renal function (C-III).
Recommendation 2. If there is no evidence of proteinuria at initial evaluation, patients at high risk for the development of proteinuric renal disease (i.e., African American persons, those with CD4+ cell counts <200 mL or HIV RNA levels >4000 copies/mL, and those with diabetes mellitus, hypertension, or hepatitis C virus coinfection) should undergo annual screening (B-II). Renal function should be estimated on a yearly basis to assess for changes over time (B-II).
Recommendation 3. Additional evaluations (including quantification of proteinuria, renal ultrasound, and potentially renal biopsy) and referral to a nephrologist are recommended for patients with proteinuria of grade ⩾1+ by dipstick analysis or GFR <60 mL/min per 1.73 m2 (B-II).
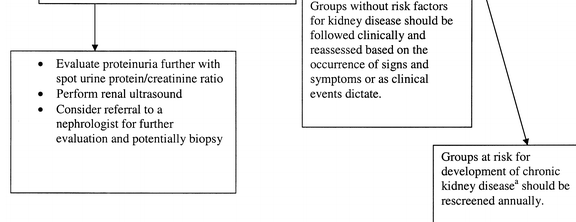
Screening for CKD. HIV infection appears to be a risk factor for developing CKD. Although no studies have examined the utility of systematic screening for early kidney disease in preventing progression of renal dysfunction in HIV-infected patients, there is evidence that early treatment of CKD is beneficial [15, 52, 57-61]. The clinical evaluation of patients at increased risk for CKD includes assessment of markers of kidney damage, such as proteinuria, kidney function, and blood pressure. Even in patients with normal kidney function, the presence of proteinuria may indicate early kidney disease. If initial urine analysis results are normal, annual follow-up urine analyses are recommended to screen for newly developed kidney damage for the following groups, which are at higher risk for the development of proteinuria and poor renal outcome: African American persons, patients with diabetes, patients with hypertension, patients with hepatitis C virus coinfection, and patients with HIV RNA levels >4000 copies/mL or absolute CD4+ lymphocyte counts <200 cells/mL [1, 2, 42]. An estimate of creatinine clearance or GFR should also be annually performed to screen for renal dysfunction that may develop over time and that may herald worse overall prognosis [3] and to stage the patient's condition, as outlined in table 2. A suggested algorithm for screening is found in figure 1.
Diagnostic evaluations. If proteinuria of grade >1+ (which roughly correlates to a protein level of 30 mg/dL or a protein-to-creatinine ratio >300 mg/g [62]) is present on screening urine analysis, then quantifying urine protein excretion using spot urine albumin-to-creatinine or protein-to-creatinine ratios provides information relevant to both type and activity of renal disease. In patients with evidence of CKD, imaging of the kidneys via ultrasound or other modality may provide information on the presence of stones, extrarenal and intrarenal lesions, and kidney size. Although HIVAN is associated with large echogenic kidneys [63], this relationship is not sufficiently predictive of HIVAN to use as a diagnostic tool. The utility of ultrasound is that patients with small kidneys (i.e., <9 cm in length) may have kidney disease that is advanced and irreversible. Additional studies usually performed to identify the cause of CKD are serological tests for hepatitis B and C, tests to determine complement levels, antinuclear antibody testing, testing to determine levels of cryoglobulin in serum, quantitative immunoglobulin testing, serum and urine protein electrophoresis testing, and testing to determine levels of glucose in serum.
Referral to a nephrologist is recommended for the evaluation of etiological results and/or the treatment of CKD. Although all decisions, including the timing of referral, should be made on a case-by-case basis, indications for such referral include abnormal kidney function, declining kidney function, or proteinuria [5]. Indications for renal biopsy in patients with HIV infection should be the same as in patients without HIV infection, including significant proteinuria, evidence of progressive disease (such as increasing proteinuria or decreasing GFR), unexplained ARF or subacute renal failure, or an acute nephritic syndrome (e.g., hematuria, proteinuria, or hypertension with renal insufficiency) [64]. Because clinical diagnosis on the basis of markers such as CD4+ cell count, HIV RNA level, and degree of proteinuria may not predict histological diagnosis in HIV-infected patients [20, 36] and because treatment options and prognosis may be influenced by the actual histological diagnosis [19], renal biopsy is recommended whenever feasible. There is no evidence to suggest that patients with HIVAN experience risk related to biopsy that is different from that experienced by patients with CKD who are not HIV infected.
MANANGEMENT
Recommendation 1. In HIV-infected patients with evidence of nephropathy, blood pressure should be controlled to a level no higher than 125/75 mm Hg (B-III), with the initial preferential use of ACE inhibitors or ARBs for those patients with proteinuria (B-II). Calcium channel blockers should be avoided in patients receiving protease inhibitors (D-II).
Recommendation 2. Dialysis and the placement of arteriovenous fistulae (native fistulae preferred [A-II]) should not be withheld for patients solely because of HIV infection (A-II).
Recommendation 3. Renal transplantation may be considered for patients with ESRD if provided in a supervised clinical trial or at centers with adequate experience in this area (C-III).
Recommendation 4. Patients with HIVAN should be treated with HAART at diagnosis (B-II). HAART should not be withheld from patients simply because of the severity of their renal dysfunction (B-III).
Recommendation 5. Addition of ACE inhibitors, ARBs, and/or prednisone should be considered in patients with HIVAN if HAART alone does not result in improvement of renal function (B-II).
General measures. Hypertension is both a cause of CKD in the general population and a proven risk factor for faster progression towards dialysis. The prevalence of hypertension in unselected HIV-infected patients is 12%-21% [65, 66]. Current guidelines from the National Kidney Foundation, which have not been specifically validated in patients with HIV-related kidney diseases, recommend a target blood pressure of 125/75 mm Hg or less, as tolerated, for patients with diabetes mellitus, proteinuria, or reduced kidney function; alternatively, the recommended blood pressure goal is 135/85 mm Hg [5]. Nonpharmacologic strategies, especially salt restriction, should be encouraged. Blockade of the renin-angiotensin system may have specific benefits in those hypertensive patients with proteinuria (see the subsection "ACE inhibition" in the section Management). Calcium channel blockers of both the dihydropyridine and nondihydropyridine classes should be used with caution because of their potential interaction with protease inhibitors, which can result in hypotension and possibly in conduction delays [67]. Dietary protein restriction is unproven and should only be attempted under close dietetic supervision.
Dialysis. For those patients with ESRD, the epidemiological data [22, 24, 45-49, 68, 69] support the use of dialysis, because HIV-infected subjects requiring either hemodialysis or peritoneal dialysis and receiving HAART are achieving survival rates comparable to those of dialysis patients without HIV infection. Renal replacement therapy can be safely delivered to HIV-infected patients by adhering to the following CDC recommendations [70]. The choice of dialysis modality between hemodialysis and peritoneal dialysis is not a factor in predicting survival among HIV-infected patients with ESRD [71].
The progression of HIVAN to ESRD can be quite rapid, and thus HIV-infected patients with CKD who have chosen hemodialysis should be referred early to a nephrologist and to a surgeon for placement of a native arteriovenous fistula. Native arteriovenous fistulae are the preferred types of access because of excellent patency once established and lower complication rates, compared with those associated with other access options. Studies have shown that thrombus-free survival for native arteriovenous fistulas in HIV-positive patients is comparable to that reported for HIV-negative patients, and infection rates associated with fistulas are lower than those associated with synthetic grafts [72-74]. Patients may need tunneled cuffed catheters for hemodialysis until maturation of the native arteriovenous fistula. HIV infection is not a significant risk factor for tunneled cuff catheter-associated infection, but there may be a higher prevalence of gram-negative bacterial infections among HIV-infected patients [75].
The incidence and spectrum of peritonitis has been reported in several small series of HIV-infected patients receiving peritoneal dialysis. The largest series studied 39 HIV-infected patients receiving peritoneal dialysis and found a higher overall risk of peritonitis and more cases of peritonitis attributed to pseudomonas species and fungi than in other patients with ESRD [49, 69]; these studies were performed prior to the availability of HAART. The higher peritonitis rate in the study could have been due to HIV infection, low socioeconomic status, and/or injection drug use. HIV has been identified in peritoneal dialysate fluid, which should be handled as a contaminated body fluid [76]. Peritoneal dialysis patients should be instructed to pour dialysate into the home toilet and to dispose of dialysate bags and lines by tying them in plastic bags and disposing of the plastic bags in conventional home garbage [77].
Transplantation. Deceased donor and living donor kidney transplantation is available for HIV-infected patients through clinical trials or as part of routine care at several transplant centers in the United States. Transplantation had not been widely available to this population until recently because of the potential risks of immunosuppression in the context of HIV disease and HIV-associated mortality itself. Prior to the use of effective antiretroviral therapy, case reports and series reported mixed outcomes in HIV-infected renal transplant recipients, including both rapid HIV progression and long-term survival [78, 79]. With the dramatic reductions in HIV-associated morbidity and mortality observed since the availability of HAART, the safety of immunosuppression in this population has become the more pressing concern. In fact, immunosuppression may have a beneficial impact on patients with HIV infection [80-82] by reducing the pool of activated T cell targets for new infection, decreasing the immune activation characteristic of HIV pathogenesis [83], inhibiting HIV replication [84], and/or interacting synergistically with antiretroviral agents [85, 86].
Preliminary short-term data in case reports and small cohorts of liver, kidney, and heart transplant recipients suggest that patient survival rates may be similar to those in HIV-uninfected transplant recipients, implying that immunosuppression may not be uniquely dangerous in the context of HIV infection [87, 88]. Furthermore, a recent analysis of the USRDS confirmed that mortality in patients receiving cadaveric kidneys in the HAART era had improved dramatically, although black patients tended to be underrepresented [89]. However, surprisingly high rates of acute and chronic rejection have been observed among HIV-infected kidney transplant recipients, with uncertain etiology at this time [88, 90]. A National Institutes of Health-funded, multicenter study of the safety and efficacy of kidney and liver transplantation in HIV-infected patients is expected to enroll patients through 2007 and to have a follow-up period of 2-5 years. Because solid-organ transplantation in HIV-infected patients is complicated by drug interactions and a complex set of infectious, metabolic, and neoplastic complications related to each condition, clinical management must be provided by a multidisciplinary team of providers who are able to communicate rapidly about evolving signs, symptoms, and laboratory abnormalities.
Antiretroviral therapy. Because HIV infection itself appears to be the cause of HIVAN and may contribute to other renal diseases in HIV-infected patients (e.g., immune-complex glomerulonephritides), antiretroviral therapy is a logical choice as a therapy for HIV-related renal diseases. Several reports have suggested limited benefits in renal outcomes associated with zidovudine monotherapy [59, 91, 92] and more-substantial benefits associated with the use of HAART [15, 57, 93-96]. In another analysis, patients who received a protease inhibitor-based regimen, compared with those who either did not receive antiretrovirals or only received a regimen of nucleoside analogues, had a lower rate of decrease in creatinine clearance (0.08 vs 4.3 mL/min per month; P = .04) [52]. An additional renal biopsy has demonstrated dramatic improvements in renal histological findings in association with HAART [15]. Unfortunately, after several years of HAART therapy, some patients eventually progress to ESRD through uncertain mechanisms [97]. In addition to being effective in treating established HIVAN, HAART may also potentially decrease the actual incidence of de novo HIVAN [27, 53].
ACE inhibition. ACE inhibition has been shown to be protective in a transgenic mouse model of HIVAN [98] and is associated with improved outcomes in several small observational studies involving humans [59, 61, 99]. Its potentially beneficial effects may be related to improved renal hemodynamics, reduced proteinuria, or cytokine modulation. In a case-control study, among 18 patients with biopsy-proven HIVAN, 9 captopril-treated patients had significantly longer mean (±SD) renal survival (156 ± 71 days) than did 9 nontreated subjects (37 ± 5 days) [59]. In a follow-up report of an earlier, nonrandomized study [99], the observed median duration of renal survival was 479 days, with only 1 case of ESRD among 28 fosinopril-treated subjects, compared with 146.5 days among the 16 subjects who declined fosinopril (P < .001), with all 16 cases progressing to ESRD [61]. An important consideration in the interpretation of these studies is that each is limited in the ability to identify and control for the potential benefits of antiretroviral medications. A substantial proportion of patients followed-up in each of these studies either did not receive antiretroviral medications or received only monotherapy, which is consistent with the standard of care available when the patients were treated. The resulting inferences are therefore difficult to generalize and are highly susceptible to selection and allocation bias. Although these data support benefit from ACE inhibitors in the absence of antiretroviral therapy or treatment with a single antiretroviral, given the proposed pathobiology of HIVAN and similar observational studies suggesting a benefit from HAART, additional research to assess the effect of full HAART with or without concomitant ACE inhibition on the progression of HIVAN is warranted.
The optimal intensity of therapy, the potential effects of ARBs, and the specific benefits of ACE inhibitors on HIV-related kidney diseases other than HIVAN are unknown. With these caveats, it is reasonable to initiate ACE inhibitors or ARBs as first-line therapy for HIV-infected patients with hypertension and proteinuria (defined as a spot urine protein/creatinine level >200 mg/g), as per K/DOQI recommendations [100], although this remains to be validated in randomized, controlled trials. No recommendations can be made regarding their use among patients with CKD that is not associated with hypertension.
Corticosteroids. Several studies have reported significant improvements in renal function and proteinuria for patients with HIVAN receiving corticosteroid therapy in the pre-HAART era [52, 58, 101-103]. In a large retrospective cohort analysis, corticosteroid therapy was associated with an improvement in creatinine clearance over time (+3.32 mL/min per month), compared with a deterioration (-5.57 mL/min per month) in non-corticosteroid treated subjects (P = .003) [52]. In another retrospective study, the adjusted RR for ESRD was 0.2 (95% CI, 0.05-0.76; P < .05) for 13 subjects who received prednisone, compared with 8 who did not [58]. Furthermore, the risk of serious infection and hospitalization in this study was not significantly different in the corticosteroid-treated patients, although the duration of hospitalization was significantly longer. The strongest evidence for the utility of prednisone therapy comes from a prospective evaluation of 20 consecutive patients with HIVAN who were given prednisone at a dosage of 60 mg/day for 2-11 weeks, followed by a taper over a 2-26-week period. Most of these patients had significant improvement in both renal function and proteinuria. Several patients experienced relapse after discontinuation of corticosteroid therapy, but they then improved with reinstitution of therapy. In a preliminary, retrospective study of the combination of HAART and prednisone in patients with biopsy-proven infection [103], infection rates were reportedly similar among a group of 15 subjects who were treated with HAART and corticosteroids (47%), compared with among a group of 7 patients treated with HAART alone (57%); median time to ESRD in the combination group (13 months) was markedly better than that in the group treated with HAART alone (6 months).
The optimal duration and intensity of therapy is unknown. Before therapy, underlying infection should be actively ruled out (B-III) and would be a contraindication for immunosuppressive therapy. However, recent data suggest that short-term prednisone therapy given as part of initial therapy for HIV infection itself—especially when combined with HAART and even in those with CD4+ cell counts of <200 cells/mm3—is relatively safe and does not predispose to severe infection [104, 105]. However, the combination of full HAART with short-term prednisone therapy has not been studied in patients with HIVAN. Patients with HIVAN whose kidney function deteriorates despite use of HAART and who are without active infection or active illicit injection drug use (or patients who are considered to be potential transplant candidates) should receive consideration of prednisone therapy at 1 mg per kg of body weight per day (maximum dosage, 80 mg/day) for 2 months, followed by a 2-4 month taper.
Non-HIVAN renal disease. The available evidence regarding therapy of HIV-associated renal diseases other than HIVAN is even more anecdotal than that described above. Several case reports have described successful treatment of a heterogeneous group of HIV-related glomerulonephritides with antiretroviral therapy and/or corticosteroids [51, 106-108]. No specific therapeutic recommendations are possible at this time.
ANTIRETROVIRAL DOSING AND RENAL TOXICITIES
Recommendation 1. Appropriate reduction of dosing for antiretrovirals that are primarily renally eliminated is warranted (C-III), with additional doses given after hemodialysis for those drugs that are readily removed by dialysis (B-II).
Recommendation 2. Nucleoside analogues should not be withheld in patients with reduced renal function for fear of the development of lactic acidosis (D-III).
Recommendation 3. Patients receiving indinavir should drink at least 1.5 L of water daily to prevent stone formation (B-III). Periodic monitoring of renal function and pyuria should be performed during the first 6 months of indinavir therapy and biannually thereafter (B-II), although routine screening for crystalluria is not warranted unless there is a suspicion of nephrolithiasis (B-II). Indinavir need not be withheld from patients with reduced renal function (C-III). In patients who develop indinavir nephrolithiasis, it would be reasonable to restart indinavir therapy once rehydration is achieved (B-III). Patients who develop indinavir-induced hypertension, pyuria, rhabdomyolysis, or renal insufficiency (acute or chronic) should permanently discontinue use of this drug (B-III).
Recommendation 4. Patients receiving tenofovir who have a GFR <90 mL/min per 1.73 m2, patients receiving other medications eliminated via renal secretion (e.g., adefovir, acyclovir, ganciclovir, or cidofovir), patients with other comorbid diseases (e.g., diabetes or hypertension), or patients receiving ritonavir-boosted protease inhibitor regimens should be monitored at least biannually for measurements of renal function, serum phosphorus, and urine analysis for proteinuria and glycosuria (B-III).
HIV Antiretroviral Dosing Recommendations
A summary of dosing recommendations for patients with CKD/ESRD is provided in table 3 (not included here). Although reduced dosing of several antiretrovirals is advised for the patient with renal dysfunction, it should be noted that there is little clinical evidence that this actually prevents the development of toxicities while maintaining full virologic efficacy. For instance, lamivudine given in normal doses to patients with renal insufficiency does not seem to cause any obvious toxicities [119, 154]. However, because of the increasing concern for drug-drug interaction in patients with renal insufficiency [155] that may not be anticipated without more clinical data, reduced dosing is still advised.
The need to adjust the dosing of antiretrovirals in the patient with CKD is underappreciated by HIV caregivers [20]. Because the nucleoside and nucleotide reverse-transcriptase inhibitors (NRTIs) are primarily excreted by the kidneys, reduced dosage is required in those with impaired renal function, especially for drugs like didanosine and stavudine, which require further reduction because their pharmacokinetics are influenced by weight. Furthermore, because the medications in this class are neither tightly protein-bound nor have a high molecular weight, they may be easily removed by dialysis. Therefore, NRTIs in general should be administered after dialysis (although extra dosing to supplement the potential loss during dialysis is usually not required). The exception to this is abacavir, which has relatively low urinary excretion, is more tightly protein-bound, and has more-extensive hepatic metabolism. Therefore, dosing adjustment for this drug in patients with renal insufficiency is not necessary, although it should still be administered after hemodialysis to minimize drug loss [120]. Providers are advised to reduce the dose of lamivudine in patients with CKD, but somewhat higher doses may be given (e.g., 100-mg tablets may be given instead of 25-50-mg tablets to avoid prescribing the drug in liquid form if this suits patient preference or if this formulation is not readily available). It should also be noted that the lower drug dosing recommendations for tenofovir are intended for patients with stable CKD and not for those whose renal function deteriorates as a result of tenofovir-related nephrotoxicity or some other acute insult. Data on the safety and efficacy of tenofovir in HIV-infected patients with creatinine clearances of <50 mL/min are currently unavailable, although studies are underway to address this important question.
On the other hand, the nonnucleoside reverse-transcriptase inhibitors (NNRTIs), protease inhibitors (PIs), and fusion inhibitors are, in general, much more tightly bound to plasma proteins and are primarily metabolized by the liver. Furthermore, the NNRTIs and PIs have high molecular weights and are excreted into the urine in low amounts. These empirical findings suggest that dose adjustment for NNRTIs and PIs in patients with CKD is not required, although little data are available to support this general conclusion. Nevirapine and indinavir, however, do not fully share the drug properties of the other NNRTI and PI medications. Because of nevirapine's relatively low molecular weight and protein-binding fraction, it has been suggested that dialysis may remove substantial amounts of this drug [133, 134]. Therefore, a 200-mg dose of nevirapine should be administered after dialysis [131]. Indinavir's plasma protein-binding fraction is also lower than those of other PIs, and 10% of unchanged drug is excreted in the urine. However, several case reports have demonstrated no appreciable change in indinavir's pharmacokinetic parameters, although it may be prudent to initiate hemodialysis at the end of a dosing interval [140, 141]. Dose adjustments for ritonavir-boosted PI combinations should not be necessary, but this also has not been fully evaluated.
Renal Dosing of Antibiotics Commonly Used in HIV Care
Many of the antimicrobials commonly used to prevent and treat opportunistic infections such as Pneumocystis jiroveci pneumonia, Toxoplasma encephalitis, and Mycobacterium avium intracellulare infections require dose reduction in the HIV-infected patient with CKD because of their renal elimination. Recommended dosing data for these drugs are available in table 4 (not included here). Of note, trimethoprim and pyrimethamine reduce renal secretion of creatinine and may subsequently cause elevation of the serum creatinine level even without actual decrement in renal function; in this situation, the clinician is advised to estimate creatinine clearance using a 24-h urine collection (rather than an estimating formula) to make decisions regarding dose adjustments. If dapsone is chosen for Pneumocystis prophylaxis in patients requiring hemodialysis, the dose should be adjusted to 50 mg po bid, with at least 1 of the doses given after dialysis (David P. Jacobus, personal communication).
Renal Effects of Commonly Used Medications in HIV Care
Nonantiretrovirals. Several drugs used in the treatment of HIV-infected persons may cause ARF. Amphotericin B, cidofovir, foscarnet, pentamidine, and high-dose acyclovir have known nephrotoxic potential and should be administered under close supervision. Amphotericin B causes renal effects in up to 80% of treated patients, including hypokalemia, bicarbonaturia, renal tubular acidosis, decreases in renal erythropoietin and anemia, and elevations in the serum creatinine level. Lipid-associated formulations of amphotericin B are less nephrotoxic than conventional amphotericin B; one of the indications for their use is for patients who develop elevations in serum creatinine level (above 2.5 mg/dL) while receiving conventional amphotericin B [156]. Cidofovir causes dose-dependent nephrotoxicity (including glycosuria, bicarbonaturia, phosphaturia, polyuria, and nephrogenic diabetes insipidus) that is reduced by coadministration of intravenous hydration and probenecid, which prevents the active uptake of cidofovir at the basolateral surface of the proximal tubule [157]. Cidofovir is contraindicated in patients with preexisting creatinine clearance of <55 mL/min or a urine protein level >2+ (100 mg/dL) on urine dipstick. Urine protein and serum creatinine levels should be monitored within 48 h before each dose; cidofovir should be discontinued if the serum creatinine level increases by >0.5 mg/dL above baseline values or if a urine protein level of >3+ develops. Renal toxicity is the major side effect of foscarnet use and can be reduced with intravenous saline or 5% dextrose solution hydration before and during slow infusion. Foscarnet administration is associated with hypocalcemia, hypophosphatemia, hyperphosphatemia, hypomagnesemia, and hypokalemia. Impaired baseline renal function, low total serum calcium level, and underlying CNS conditions were risk factors associated with seizures during foscarnet treatment. Determintion of creatinine clearance and measurement of electrolytes are recommended at baseline, 2-3 times per week during induction therapy, and every 1-2 weeks during maintenance therapy. Acyclovir, ciprofloxacin, foscarnet, and sulfonamides can cause intratubular precipitation of crystals leading to ARF; avoidance of rapid intravenous bolus and hydration are recommended, as well as adjustment for renal dysfunction [158].
Antiretrovirals. One study has suggested an association between reduced kidney function (creatinine clearance, <70 mL/min) and the development of lactic acidosis in patients receiving NRTIs [159], although withholding this valuable class of medications for fear of a relatively uncommon side effect seems unwarranted without further long-term study. Although ritonavir [160, 161] has occasionally been reported to cause ARF, most documented cases of ARF due to currently used antiretrovirals are due to indinavir and tenofovir.
Indinanvir. Nephrolithiasis is the major side effect of indinavir therapy [162, 163]. Although it is mainly metabolized (80%) in the liver, its pH-based solubility in urine makes renal excretion extremely important. Kopp et al. [164] reported that indinavir crystalluria is associated with a novel syndrome that consists primarily of back or flank pain and renal parenchymal filling defects on CT scan but without nephrolithiasis. They also reported symptomatic patients with crystalluria, dysuria, and urgent urination; asymptomatic crystalluria occurred in 20% of indinavir-treated patients. Pyuria (but not necessarily crystalluria) secondary to treatment with indinavir has been associated with gradual loss of renal function unrelated to obstructive symptoms [165]. Risk factors include low lean-body mass, indinavir regimens of ⩾1000 mg administered twice daily, concomitant use of trimethoprim-sulfamethoxazole [163, 166], and coinfection with either hepatitis B or C [167, 168]. Environmental conditions, including temperature, atmospheric pressure, and humidity, affect the risk of developing indinavir nephrolithiasis [163, 169]. Ritonavir-boosted indinavir regimens may lead to higher risk of nephrolithiasis, with higher indinavir peak concentrations being associated with development of urologic symptoms [170], as well as to rhabdomyolysis-induced renal failure [171]. In addition, the highest incidence of urologic symptoms occurred during the first 6 months of indinavir treatment. Symptoms continue to occur after this period but at a slower rate [163]. Fortunately, most cases of ARF secondary to indinavir resolve with discontinuation of the drug. On suspicion of indinavir nephrotoxicity, urine should be inspected for the presence of crystals and pyuria [172]. Indinavir has also been associated with hypertension [173], renal atrophy [174], interstitial nephritis [175, 176], development of renal failure associated with fever and rash [177], and persistent leukocyturia with renal failure [178, 179]. A daily intake of at least 1.5 liters of water usually prevents stone formation, especially in those with the risk factors outlined above. There is little risk of recurrent nephrolithiasis or ARF associated with dehydration if indinavir therapy is restarted once rehydration is achieved. Indinavir should not be withheld in patients with severe kidney disease for fear of increased risk of nephrolithiasis because little of the drug will actually reach the collecting system. For patients who develop recurrent indinavir nephrolithiasis thought to be due to high drug levels secondary to ritonavir-boosting effects, there is no evidence that restarting indinavir therapy without ritonavir therapy (i.e., indinavir administered at 800 mg every 8 h) in patients without other antiretroviral options results in recurrent nephrolithiasis. It should be noted that renal adverse effects may occur during long-term indinavir therapy or after discontinuation of indinavir therapy [180].
Tenofovir. Proximal renal tubule toxicity has been described in patients receiving the antiviral acyclic nucleoside phosphonate class of medications, including adefovir and cidofovir. These drugs are excreted primarily as unchanged drug in the urine. Renal toxicity occurs with accumulation of these compounds in the proximal tubule and appears to be concentration dependent [181]. When administered at doses required for HIV treatment [182], adefovir dipivoxil caused unacceptably high rates of nephrotoxicity characterized by the Fanconi syndrome (renal failure, hypokalemia, hypophosphatemia, metabolic acidosis, albuminuria/proteinuria, hyperaminoaciduria, glucosuria, calciuria, and phosphate and potassium wasting). However, at the lower dose approved for the treatment of hepatitis B virus infection, renal failure rates appear low [183, 184]. Tenofovir was not associated with higher rates of renal failure in initial treatment studies [185, 186], although patients with preexisting renal dysfunction were not included. Accumulating cohort study data for tenofovir in general use have shown quite low incidence rates of renal failure, on the order of 0.5%-1.5% [187, 188]. However, several case reports and small case series of patients developing Fanconi syndrome after initiating tenofovir-containing regimens (which then resolved or improved with discontinuation of the use of tenofovir) have been and continue to be published [189-195], suggesting that there may be specific risk factors for the few patients who develop tenofovir-related nephrotoxicity.
Most of the cases described in the literature occurred in subjects who were receiving prolonged courses of tenofovir plus ritonavir-containing combination therapy (including lopinavir, saquinavir, atazanavir, and amprenavir) salvage regimens [189, 190, 192, 193]. Pharmacokinetic analysis showed that tenofovir exposure was increased by 32% when administered with lopinavir/ritonavir therapy, compared with when administered alone [196]. It is postulated, although not proven, that ritonavir blocks the MRP-2 transporter on the apical side of the kidney proximal tubule, and thus prevents excretion of intracellular tenofovir into the urine [189]. The higher concentration of tenofovir that results may then cause proximal tubule necrosis and denuding of the basement membrane. Data from an ongoing study of tenofovir administered in combination with lopinavir/ritonavir [197] suggest that renal toxicity involving the proximal tubule occurred in only 1 of 190 subjects during the first 48 weeks of therapy; notably, this subject had a baseline creatinine clearance of 40 mL/min and was receiving full-dose tenofovir therapy. Alternatively, the association between the use of tenofovir with ritonavir-boosted protease inhibitors and Fanconi syndrome may simply be a marker of patients in deep salvage therapy with more longstanding HIV infection that could, in general, lead to a higher incidence of nephropathy. This is supported by a cohort study [188] that suggests that renal toxicity occurs primarily in patients with more-severe HIV disease and longer overall antiretroviral treatment duration. Reynes et al. [198] further reported, in their series of 74 patients receiving tenofovir therapy, that Fanconi syndrome developed in 3 patients, with an estimated incidence of 4 cases per 100 patient-years of tenofovir treatment. Risk factors in this study included preexisting renal dysfunction, low body weight, and long duration of tenofovir use [194]. Prior exposure to high-dose adefovir dipivoxil does not appear to be associated with an increased risk of nephrotoxicity [187]. As is the case with indinavir, tenofovir-induced renal failure appears to improve with discontinuation of treatment with the drug.
HIV INFECTION AND CKD IN THE PEDIATRIC AND ADOLESCENT POPULATIONS
Recommendation 1. In children without evidence of existing renal disease, screening evaluation for the development of HIVAN is similar to that proposed earlier for adults and should include complete urinalysis and testing to determine serum electrolyte levels, blood urea nitrogen levels, and creatinine levels every 6 months (C-III).
Recommendation 2. Pediatric HIVAN and other proteinuric nephropathies in HIV-infected children should be treated with HAART; referral to a nephrologist and the addition of ACE-inhibition should also be considered for patients with more-severe proteinuria (grade ⩾1+ by urine dipstick analysis or a protein-to-creatinine ratio ⩾0.2 g/g for 3 separate specimens) (C-III). Steroid use is not recommended for this population (D-II).
Epidemiology. The 2002 CDC HIV/AIDS Surveillance Report estimated that there were 3219 pediatric cases of HIV infection in American children <13 years of age, with 41,000 infected patients between the ages of 13 and 24 years. These numbers were generated to reflect cases in 30 areas with required reporting of HIV infection and are likely to be an underestimation of infection nationwide. The incidence of HIV-associated kidney disease in these children is estimated to be 2%-5% [199-201]. In addition, 5% of deaths in HIV-infected children are reported secondary to kidney disease [202]. The Pediatric AIDS Clinical Trials Group (PACTG) has attempted to estimate the prevalence of renal disease in pediatric patients with HIV infection. Analysis of the PACTG 219C Late Outcomes Study databases (Russ Van Dyke, unpublished data) suggests that 2%-3% of subjects have a renal diagnosis consistent with HIV nephropathy and that up to 6% may have renal disease as determined principally by laboratory evaluation. Of 145 subjects, 48% had hypokalemia, 33% had elevated blood urea nitrogen levels, 17% had elevated creatinine levels, and 14% had low serum albumen values. Proteinuria was detected on >2 occasions in 29% of subjects. Among subjects in the PACTG database, 44 patients, most of whom were black, had diagnoses consistent with HIV nephropathy. Concerns exist that these numbers underestimate the prevalence of renal disease because of the possibility that there are other children with moderate-to-severe renal disease being cared for at clinical sites who have never been enrolled in a PACTG study (Warren Andiman, personal communication).
In-progress analysis of a pediatric database at the University of Miami demonstrates that 77 of 284 HIV-infected children included in the database were found to have persistent proteinuria, defined as urinary protein-to-creatinine ratios of >0.2 g/g. Another 34 children had HIVAN, defined according to the clinical criteria of persistent proteinuria plus changes in radiographic or scintigraphic findings consistent with the diagnosis. Of these 34 children, the nephrotic syndrome developed in 7 (21%), and 14 (42%) progressed to chronic renal insufficiency. The severity of HIVAN correlated with persistently high viral loads, although 1 patient developed overt HIVAN despite good viral control (C.D. Mitchell, unpublished data). The University of Miami database, analyzed by retrospective chart review from January 1998 through January 2001, determined that 13.6% of pediatric HIV-infected patients who were receiving HAART had HIVAN; these data were not appreciably different from the reported pre-HAART prevalence of 10%-15% [203].
Progression to ESRD in children is highly dependent on the histopathological diagnosis. FSGS denotes a poor prognosis, with rapid progression to ESRD within 1 year after presentation. There is a high mortality rate associated with the FSGS diagnosis, with cause of death usually unrelated to renal disease [204]. In one report, the median time from clinical detection of nephropathy to severe renal failure was 9 months (range, 1-27 months) [205]. Another report compared children with AIDS with or without associated nephropathy. All children with AIDS and nephropathy (n = 16) died during the 10-year study period, with a mean survival time of 9.5 months after renal disease was diagnosed. Thirty-two (70%) of 56 children without nephropathy were alive at the end of the study period. FSGS was the most common renal lesion in this series, diagnosed in 6 of 13 children who underwent renal biopsy [206]. A report describing the experience in the Washington, D.C., area from 1985-1997 determined a mortality rate of 82% among their pediatric patients with HIVAN during the study interval; the majority died before reaching ESRD [200]. Of 15 children with nephrotic syndrome who were followed-up from 1984 through 1990 in Brooklyn, New York, 30% experienced ESRD within 8 months after diagnosis, with successful peritoneal dialysis performed for 3 children. However, 80% died from HIV-related complications during the study interval [201]. A recent report by Ahuja et al. [207] analyzed the USRDS database to evaluate prevalence and survival of children with HIVAN in the U.S. Only 60 (0.78%) of the reported 7732 patients with HIVAN were <21 years of age. Of these children, 88.3% were black, and 50% were male. Survival rate for children in this database was better than that for adults with HIVAN at 12, 24, and 36 months (76%, 62%, and 54% for children, compared with 60%, 43%, and 34% for adults). The major factor associated with survival was female sex. The authors concluded that only a small number of children with HIVAN and ESRD have received dialysis in the United States and that prognosis for children with HIVAN is better than for adults with HIVAN.
Clinical presentation. In children, as in adults, proteinuria may be the earliest clinical presentation of HIVAN and may rarely be the first manifestation of HIV infection in a patient with unsuspected disease [208]. The degree of proteinuria may vary from minimal to nephrotic-range proteinuria [204], with associated clinical findings of edema and the full nephrotic syndrome. Ingulli et al. [201] reported that 15 children from their cohort of 164 pediatric AIDS clinic patients developed nephrotic syndrome. Five of these patients experienced ESRD by 8 months after diagnosis [201]. One series that described 6 pediatric patients with HIVAN reported that 15 patients presented with nephrotic-range proteinuria, and 1 presented with mild proteinuria. Two of these patients had hematuria in addition to proteinuria, and 1 patient received a diagnosis of renal tubular acidosis [206]. Renal disease in children who are HIV positive may be "classic" HIVAN, but it may also include fluid and electrolyte abnormalities, urinary tract infections, renal tubular acidosis, ARF, treatment-related nephrotoxicity, infiltrative diseases of the kidney, hemolytic uremic syndrome, or IgA nephropathy [204, 209]. In a situation similar to that for adults, persistent sterile leukocyturia has been reported in children receiving indinavir, accompanied by reversible impairment in renal function [210].
The findings of ultrasound evaluation of the kidneys may be normal; however, echogenic kidneys that are large for the patient's age and height may be seen at early and late stages of HIVAN [204]. Nuclear renal scans utilizing mercaptoacetyltriglycine scintigraphy have been described as showing diffuse parenchymal dysfunction [211]; however, this abnormality is nonspecific and not pathognomonic for HIVAN.
Screening and evaluation. A brief review of normal renal function in the pediatric population may assist the clinician in recognizing abnormal findings in children, for whom normal laboratory values vary by age. Although it is at times inaccurate, estimation of GFR in clinical practice can be easily performed with use of a formula derived by Schwartz et al. [212, 213]. Schwartz and coworkers devised a useful graph (figure 2) for mean normal creatinine values by age, based on applying this formula to a large population of healthy children [213]. An online calculator is also available at the Web site of the National Kidney Disease Education Program [214].
Compared with adults, more-frequent screening is required for children because of the laboratory changes associated with growth and development. If proteinuria is detected, a urinary protein-to-creatinine ratio measurement is indicated (normal ratio, <0.2 g/g) [215, 216]. Additional investigations may include a complete metabolic panel, including determination of total protein and albumin levels; serological testing for hepatitis B, C3, and C4; antinuclear antibody testing; or urine cultures for bacteria or viral pathogens. Timed urine collections for protein excretion and creatinine clearance measurements may be indicated in children who are toilet trained. Renal sonogram may be helpful if hematuria, infection, or renal insufficiency is present.
Referral to a pediatric nephrologist is warranted for persistent significant proteinuria (grade >1+ by urine dipstick analysis or protein-to-creatinine ratio >0.2 for 3 specimens), persistent microscopic hematuria, gross hematuria in the absence of urinary tract infection, edema, hypertension, recurrent urinary tract infections, electrolyte abnormalities, persistent metabolic acidosis, or elevated blood urea nitrogen or creatinine levels. Persistent proteinuria or renal insufficiency may be indications for percutaneous renal biopsy to determine the histopathological diagnosis and guide prognosis therapy.
Treatment. Although aggressive antiretroviral therapy has been demonstrated to improve renal function in some patients, data concerning adjustment of HAART dosing in children with renal disease is unavailable. Drugs that require elimination by the kidney should have dose adjustments made to avoid toxicities in all patients with renal insufficiency, using data for adults as a guide. The combined expertise of the infectious disease specialist and pediatric nephrologist should be employed for developing a treatment strategy for HIV-infected children with ESRD.
Because data concerning treatment of HIVAN in children are lacking, therapeutic strategies should mirror those used for adult patients. As in adults, experimental evidence had suggested a direct role for HIV infection in renal pathogenesis of childhood HIVAN [217, 218]. Therefore, treatment goals include reduction of HIV replication to slow progression of renal disease, although studies evaluating the use of HAART or angiotensin-converting enzyme inhibitors in children with HIV-related renal diseases have not been reported. In the 15 children with nephrotic syndrome described above, 13 were treated with steroid therapy, with no response. Three patients achieved remission of proteinuria with cyclosporine treatment [201]. Although clinical trials involving children have not been carried out to date, current practice may include treatment of significant proteinuria with oral ACE inhibitors, such as enalapril administered at 0.08 mg/kg of body weight per day up to 2.5 mg as a starting dose, then titrated to effect. A decrease in proteinuria of 50% is considered a sign of therapeutic effect. Data concerning outcomes of renal transplantation in HIV-infected children are lacking, although this may prove to be a viable option [204, 219].
SPECIAL TOPICS
Recommendation 1. Use of recombinant human erythropoietin should be considered in patients with hemoglobin levels 2 g/dL less than the lower limit of normal; the therapeutic hemoglobin target is a hemoglobin level of 11-12 g/dL (C-III).
Recommendation 2. Analagous to the general population with CKD, all HIV-infected ESRD patients with secondary hyperparathyroidism (serum calcium level, <9.5 mg/dL; serum phosphorus level, <4.6 mg/dL; and serum parathyroid hormone level, >35 pcg/mL) should be treated with 1,25-dihydroxy vitamin D3 or its analogues (C-III).
Recommendation 3. HIV-infected patients requiring hemodialysis should have anti-HBs titers checked after receiving a standard primary series of 3 hepatitis B vaccinations and should receive a fourth injection if these titers are <10 IU/L (B-II).
Anemia and CKD. The problem of anemia in patients with CKD due to insufficient production of erythropoietin is further compounded if patients are infected with HIV. Anemia is the most common hematological abnormality in HIV-infected patients [220]. Shrivastava et al. [221] found that the mean baseline hematocrit of HIV-infected patients with ESRD was 22%, compared with 26% in diabetic and nondiabetic patients with ESRD who did not have HIV infection. Similarly, Abbott et al. [222], using data from the Dialysis Morbidity and Mortality Wave 2 study, found that mean hematocrits (±SD) in patients with HIVAN (26.2% ± 6.5%) were significantly lower than those in all other patients with ESRD starting dialysis (30.5% ± 6.1%; P < .05). In both CKD and HIV infection, the presence of anemia is independently associated with shorter survival [220, 223].
In an effort to improve outcomes in patients with CKD and ESRD, the K/DOQI has published practice guidelines for taking care of patients with anemia and CKD [224]. The applicability and the efficacy of these guidelines in managing anemia in patients with HIV infection and CKD have not been addressed. K/DOQI recommends that testing for anemia should be initiated for patients with CKD when the hemoglobin level is <11 g/dL (hematocrit, <33%) in premenopausal females and prepubertal patients and when the hemoglobin level is <12 g/dL (hematocrit, <37%) in adult men.
Recombinant human erythropoietin therapy is an appropriate treatment option for patients with symptomatic mild anemia or moderate anemia (hemoglobin level, >2 g/dL below the lower limit of normal). The target range for hemoglobin level recommended for patients with CKD is 11-12 g/dL. Shrivastava et al. [221] found that the response to recombinant erythropoietin in HIV-infected patients with ESRD, despite the presence of coexisting opportunistic infections and the use of the antiretroviral agent zidovudine, was similar to that in HIV-negative patients. After 8 weeks of erythropoietin administered at 100 U/kg 3 times per week, the mean increase in hematocrit was 5.8%, compared with 6.7% in HIV-negative patients.
Iron is also essential for hemoglobin formation. K/DOQI recommends that iron status be monitored by the transferring saturation and serum ferritin levels; sufficient iron should be administered to maintain a transferring saturation level >20% and a serum ferritin level of ⩾100 ng/mL [224]. Measurements of iron indices are complicated in HIV-infected patients, especially because levels of ferritin, which is an acute-phase protein, are often elevated in patients with HIV infection. To achieve K/DOQI goals, administration of intravenous iron is required in the majority of patients receiving dialysis, although the safety of this form of therapy in terms of immune activation is unknown.
Renal osteodystrophy. CKD and ESRD are associated with osteodystrophy. In HIV-infected patients, several reports of osteopenia and osteoporosis have been described in the literature [225]. Although the underlying mechanisms triggering bone loss in HIV-infected patients are not completely defined, traditional risk factors, HIV infection itself, HIV-associated fat redistribution, antiretroviral therapy for HIV infection, and increased production of proinflammatory cytokines (such as TNF and IL-6) may have roles in osteoclast activation and resorption [225-230]. HIV-infected patients have been reported to have low baseline and maximal secretion of parathyroid hormone and 1,25-dihydroxyvitamin D3 levels [228, 231, 232]. Parathyroid cells have been found to express protein recognized by antibodies directed against CD4+ cells, suggesting that parathyroid cells may be infected with HIV and with subsequent impairment of parathyroid hormone release. Low 1,25-dihydroxyvitamin D3 levels are associated with low CD4+ lymphocyte counts, advanced HIV infection, and higher TNF-a levels. Abbott et al. [222] found that the mean parathyroid hormone level (±SD) was lower in patients with HIVAN and ESRD (239 ± 225 pcg/L) than in patients with other causes of ESRD (308 ± 319 pcg/L), although the difference was not statistically significant. Although data on bone disease in patients with CKD and HIV infection is not available, it is known that HIV-infected patients with CKD develop complications of altered calcium and phosphate metabolism similar to HIV-negative patients [233]. K/DOQI has also published clinical practice guidelines for bone metabolism and disease in CKD [234], although these strategies need confirmation in the HIV-infected population.
Vaccinations. The immunosuppression resulting from both HIV infection and CKD is likely to lead to suboptimal responses to vaccinations. The development of protective antibodies and the duration of this protection is likely to be short because of decreases in the levels of these antibodies [235]. Hepatitis B virus remains a significant risk to patients receiving chronic hemodialysis. Several reports have suggested that antibody response to vaccination for hepatitis B is impaired in HIV-infected patients [236, 237]. A recent review of hepatitis vaccination data for 348 HIV-infected patients who received dialysis in Gambro dialysis units in the United States from 1994-2003 revealed that only 54.3% of those who received the 3-dose series of 40-mg subcutaneous hepatitis B vaccine (Recombivax HB; Merck) developed protective anti-HBs titers >10 IU/L (T. Ahuja, unpublished data). Therefore, anti-HBs titers should be checked following vaccination for hepatitis B, and a booster dose should be offered if antibody levels are <10 IU/L. Table 5 lists the vaccinations that HIV-infected patients with CKD should be offered (adapted from [238]).
FUTURE DIRECTIONS
There is a great need for well-designed, prospective studies evaluating the natural history of kidney diseases in HIV-infected patients in both the adult and the pediatric populations. Studies of consecutively evaluated patients with evidence of renal disease that compare histological and clinical diagnoses would be invaluable. Screening strategies for incipient renal disease need to be tested rigorously. The modulating effects of hepatitis B and/or hepatitis C on renal disease and outcomes in HIV-infected patients also require investigation. Prospective, randomized controlled trials for the treatment of HIVAN and other HIV-related proteinuric renal diseases (e.g., hepatitis B- and hepatitis C-induced glomerulonephritides) are clearly required. More pharmacokinetic evaluations of the proper dosing of antiretroviral agents in both children and adults should also be performed. Some of these questions are currently being addressed by the PACTG and the Adult AIDS Clinical Trials Group, and they will hopefully lay the foundation for further research in this emerging field.
|
|