| |
Lipodystrophy: defined, risk factors, switch studies
|
| |
| |
"Redefining Lipodystrophy Syndrome: Risks and Impact on Clinical Decision Making"
JAIDS Journal of Acquired Immune Deficiency Syndromes: Volume 39(4) 1 August 2005
Lichtenstein, Kenneth A MD
From the University of Colorado Infectious Disease Group Practice, Denver, CO.
Dr. Lichtenstein serves as advisor and speaker for Abbott Laboratories, Bristol-Myers Squibb, GlaxoSmithKline, Gilead, Merck, and Boehringer Ingelheim; currently receives support for research from Bayer and Serono; and has received support from Abbott Laboratories, Merck, Chiron Corp., Bristol-Myers Squibb, and GlaxoSmithKline.
Author Conclusions: Use of the term lipodystrophy syndrome may need to be reevaluated. The components of this syndrome can occur independently, and recent data suggest that peripheral lipoatrophy and central lipohypertrophy are associated with different constellations of host, disease, and drug risk factors and may have different pathogenic pathways. Future studies with longitudinal rather than cross-sectional design will help to clarify the nature of the relationships among these factors. The continued elucidation of molecular pathways involving 11--HSD, adipokines, cytokines, and mitochondrial DNA suggests a complex disease process that is likely multifactorial. Switch studies attempting to avoid or minimize metabolic or morphologic complications tend to show some effect on adipose tissue. There is no evidence that switching from a PI-based regimen to a potentially less potent non-PI-based regimen has a significant impact on fat redistribution measures, as shown in the PIILR study. Given the growing evidence with thymidine analogues, there is greater interest in exploring the potential benefit of minimizing risk of lipoatrophy by using nucleoside backbones that do not contain a thymidine analogue.
ARTICLE TEXT
In 1998, 2 studies with dramatic implications for the management of HIV infection were published. Palella et al,1 using national surveillance data, reported a dramatic decline in HIV-related mortality, from 29.4 per 100 person-years in 1995 to 8.8 per 100 person-years in 1997, and an equally dramatic decline in opportunistic infections. These benefits were attributed to the introduction of protease inhibitor (PI)-based highly active antiretroviral therapy (HAART). Carr et al2 concurrently described a new syndrome in patients with HIV infection receiving HAART. This syndrome was characterized by fat redistribution and metabolic abnormalities (dyslipidemias and glucose disorders). Fat redistribution was a concern because the morphologic changes were viewed by many patients as stigmatizing and highly undesirable. Subsequent studies revealed a series of potential host-, disease-, and treatment-related risk factors. This review examines literature on risk factors and pathogenesis of lipodystrophy syndrome in HIV-1-infected individuals.
SUMMARY:
Lipodystrophy syndrome comprises several conditions (lipoatrophy; lipohypertrophy; mixed syndrome, often associated with dyslipidemia; and insulin resistance). These conditions, though sometimes occurring together, may occur independently, suggesting a complex, multifactorial cause.
To elucidate the relative contribution of risk factors of drug, disease, and host to fat redistribution, large epidemiologic studies using multivariate analysis were reviewed.
In studies assessing lipoatrophy, the most common statistically significant risk factors were use of specific nucleoside analogues, increasing age, presence of markers of disease severity (CD4/HIV RNA), duration of therapy, and white race. In studies assessing lipohypertrophy, the most common statistically significant risk factors were duration of therapy, markers of disease severity, and protease inhibitor use.
The pathogenesis of these disorders is complex, but recent hypotheses and evidence suggest that impairment to adipocyte differentiation, impairment of adipokine regulation, unopposed production of proinflammatory cytokines, dysregulation of 11--hydroxysteroid dehydrogenase, and mitochondrial toxicity may play a role.
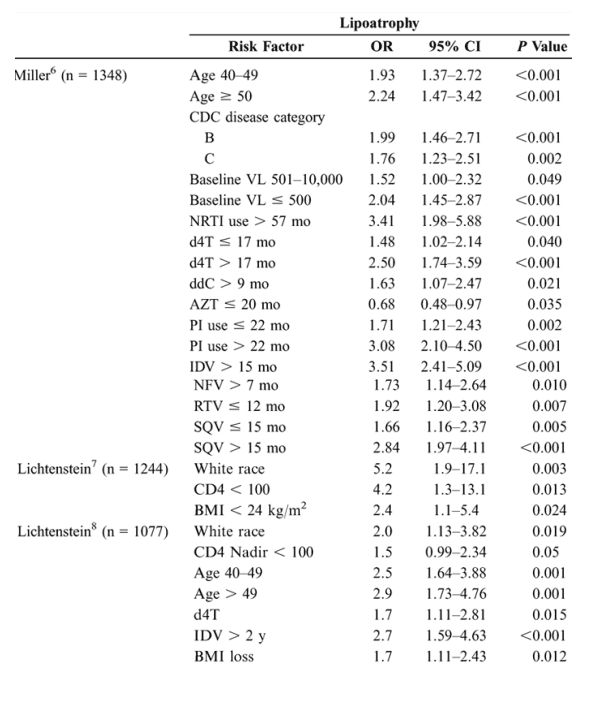
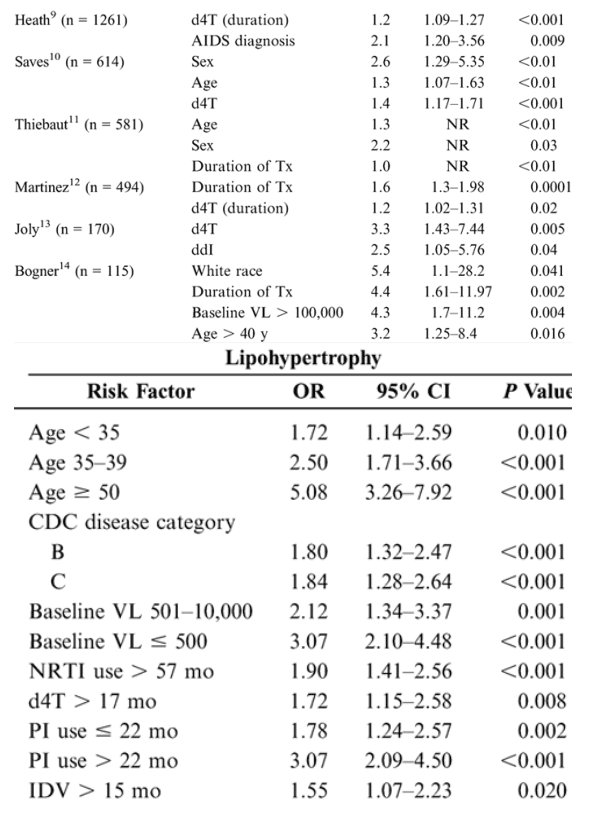
Risk Factors for Lipoatrophy and Lipohypertrophy: Multivariate Analyses
Epidemiologic studies suggest that antiretroviral therapies and nondrug factors are possible risk factors for fat redistribution. Table 1 (see below) summarizes statistically significant risk factors identified after a literature search of epidemiologic studies published or reported as of February 2005 and employing multivariate analysis using lipoatrophy and lipohypertrophy as dependent variables.
Studies that used univariate analysis or did not distinguish lipoatrophy from lipohypertrophy were excluded. The cross-sectional design of these studies limits conclusions to those of associated risk factors rather than direct causality. These data should be interpreted with an understanding of the limitations of epidemiologic studies, many of which are single-site, retrospective, nonrandomized, and subject to the phenomenon of colinearity. Colinearity occurs when a variable that is shared by a high percentage of subjects in a study sample shows an association with fat redistribution that does not necessarily exist, particularly in studies that are underpowered. A variable that occurs with a high frequency in a study sample may be associated statistically irrespective of causality. For example, we reported a prevalence study based on a survey of 1077 patients from the HIV Outpatient Study that found a highly statistically significant relationship between lipoatrophy and increasing age in the multivariate analysis.8 We subsequently conducted an incidence analysis 2 years later utilizing a 2nd survey of 1244 patients, 546 of whom were evaluated in the 1st survey.7 Of these same 546 patients, 337 (61.7%) had no signs of lipodystrophy at survey 1, but 44 of the 337 (13.1%) developed moderate/severe lipoatrophy at survey 2. When these patients were stratified by CD4 cell count at the 2nd survey, age >40 years was not statistically significant at any CD4 stratification. In fact, lower CD4 cell count at the 2nd survey was the strongest factor predicting new lipoatrophy (OR: 4.2; 95% CI: 1.34-13.07; P = 0.013). Patients whose CD4+ T-lymphocyte cell counts did not improve with treatment were also more likely to develop lipoatrophy (OR: 2.73; 95% CI: 1.14-5.35; P = 0.024). Similarly, when CD4 cell counts spanning the 6-year period prior to the 2nd survey were analyzed, low CD4 cell counts were the strongest predictor of the development of lipoatrophy. Even if significant in multivariate models, other measures of disease severity (eg, viral load, body mass index) added little predictive power. Because the 1st study measured prevalence and the 2nd measured incidence, associated risk factors differed due to the methods used and the patient populations studied.
In the 9 studies assessing lipoatrophy, the most common statistically significant risk factors were exposure to and duration of thymidine analogues-most commonly stavudine (d4T) (6/9), age (5/9), presence of markers of disease severity (CD4/HIV RNA) (5/9), duration of therapy (3/9), and white race (3/9). An additional nonrandomized, prospective study in 40 HIV-positive patients beginning their 1st antiretroviral therapy reported results after a mean of 96 weeks of therapy. In multivariate analysis, treatment with d4T was the strongest independent factor associated with rate of lipoatrophy (P = 0.05).15 In the 8 studies assessing lipohypertrophy, the most common statistically significant risk factors were duration of therapy (3/8), markers of disease severity (3/8), age (3/8), and protease inhibitor use (4/8).14 A further study in 2258 HIV-positive patients assessed adipose tissue alterations by gender.16 Logistic regression analysis demonstrated that men had a significantly lower adjusted risk of presenting with any alteration than women (OR: 0.47; 95% CI: 0.38-0.58; P < 0.0001) and a significantly lower risk of lipohypertrophy (P = 0.0022) and mixed fat redistribution (P < 0.0001), whereas risk of lipoatrophy was similar between genders.16 Thus, rigorous multivariate analyses controlling for numerous variables reveal multiple risk factors, suggesting that the pathogenic mechanisms for fat redistribution are likely the result of complex interactions between host, disease, and drug factors.
Impact of Switch Therapy on Fat Redistribution
Several recent studies have assessed the impact of altering regimens to determine the impact of drug therapy on fat redistribution. Martin et al17 recently reported the 120-week results from the Protease Inhibitor Induced Lipodystrophy Reversal Study (PIILR) in which patients with moderate-to-severe fat redistribution were randomly allocated to either stop or continue PI therapy.17,18 At 24 weeks, all patients were offered the option of stopping their PI therapy and switching to a regimen containing nucleoside reverse transcriptase inhibitors (NRTIs) adefovir and hydroxyurea. This trial included yearly DEXA and CT scans.18 At 120 weeks, a cohort of 45 patients from both arms with body composition measurements was available for analysis. Linear regression analysis of this cohort showed no association between fat redistribution indicators, weight, triglycerides, initial treatment arm, PIs, adefovir, or hydroxyurea, but a statistically significant association with thymidine analogue use and limb fat mass reduction (d4T: 0.72 kg/y, P = 0.004; zidovudine [AZT]: 0.29 kg/y, P = 0.019) and lipodystrophy score increase (d4T, P < 0.001; AZT, P < 0.001).17 Conversely, Boyd et al19 reported preliminary results on the impact of replacing an NRTI regimen with an NRTI-sparing regimen (indinavir-ritonavir-efavirenz) in a 48-week observational study of 61 patients. DEXA scans showed statistically significant increases in limb fat after 48 weeks of the NRTI-sparing regimen, and CT scans showed increases in subcutaneous and visceral fat in the abdomen (P = 0.04) and thigh (P < 0.001), suggesting a partial reversal of lipoatrophy. There was also a notable mean decrease in lean limb tissue (-955 g), suggesting that the increase in limb fat may reflect a general increase in adiposity supported by the overall increase in visceral adipose tissue.19
Preliminary data from a prospective, randomized, controlled trial (AIDS Clinical Trials Group [ACTG] Substudy 5005 of Study 384)20 assessed fat redistribution in antiretroviral-naive patients randomly allocated to 1 of 6 treatment arms: AZT/lamivudine (3TC) plus nelfinavir (arm A), efavirenz (arm B), or nelfinavir/efavirenz (arm C); or didanosine (ddI)/d4T plus nelfinavir (arm D), efavirenz (arm E), or nelfinavir/efavirenz (arm F). This study enrolled 330 patients from the 980 patients enrolled in ACTG 384. DEXA was used to objectively quantify fat redistribution over time relative to baseline (the primary outcome variable of the study), with all scans analyzed at a central location. Of 330 subjects, 156 had DEXA at entry, 127 of 156 (81%) had DEXA at week 48, and 107 of 156 (69%) had DEXA at week 64. Patients receiving DEXA were similar to those not receiving DEXA (n = 823) in terms of age, CD4 count, and body mass index, but the no-DEXA group had a lower mean viral load (4.9 vs. 5.1; P = 0.014). Limb fat increased early in patients randomly assigned to AZT-3TC plus protease inhibitor or nonnucleoside reverse transcriptase inhibitor (NNRTI), as well as in patients randomly allocated to receive ddI-d4T plus PI or NNRTI. At weeks 48, 64, and 80, patients randomly assigned to ddI-d4T had greater decrease in limb fat than those on AZT-3TC. The AZT-3TC arm had fat loss as well, although it occurred less rapidly and to a lesser extent than in the ddI-d4T arm. At week 80, patients randomly assigned to receive nelfinavir had a greater percentage loss of limb fat than those randomly assigned to the efavirenz arm; limb fat in the efavirenz arm also decreased, though to a lesser degree. The strength of this study is its long-term follow-up of a prospectively defined population. The final results of ACTG 5005 and similar trials (ACTG 5142) prospectively evaluating antiretroviral-naive populations treated with PI-, NRTI-, or NNRTI-sparing regimens will help clarify the course of fat redistribution. Recently reported data from the mitochondrial toxicity (MITOX) study demonstrated that peripheral limb fat, as assessed by DEXA and CT scans, increased over long-term follow-up after switching from thymidine analogue-based therapy to abacavir-based therapy.17 At week 104, patients who switched to abacavir showed a 1.26-kg mean increase in limb fat, relative to baseline (mean, 3.7 kg). Multivariate analysis demonstrated that the increase in limb fat was associated with less exposure to the thymidine analogues before the study (AZT, P = 0.024) or on study (d4T, P = 0.004).
Pathogenesis
Several pathogenic mechanisms for fat redistribution in HIV have been hypothesized. For lipoatrophy, these include impairment of adipocyte differentiation, adipocyte apoptosis mediated by proinflammatory cytokines such as tumor necrosis factor (TNF-), dysregulation of 11--hydroxysteroid dehydrogenase (11--HSD), and mitochondrial toxicity. Bastard et al21 showed that adipocytes from patients with lipoatrophy treated with NRTIs and PIs have higher levels of sterol-regulatory-element-binding-protein-1 (SREBP1c, an adipocyte transcription factor) and higher expression of TNF- than do adipocytes from healthy controls. Furthermore, adipocytes in patients with HIV were smaller and tended to cluster, suggesting an impairment of differentiation despite the increased amount of SREBP1c protein; the investigators posited that the transcription factor for SREBP1c was sequestered in an inactive form in the nuclear membrane, a phenomenon seen in vitro when adipocytes are treated with protease inhibitors.21
Lipoatrophic tissue from HIV patients showed increased expression of TNF-, a cytokine known to induce apoptosis of adipocytes.21 In a study of 56 subjects who had aspirations of subcutaneous adipose tissue, Johnson et al22 also found elevated TNF- expression in HIV-positive patients with fat redistribution (n = 28) compared with HIV-positive patients who did not have fat redistribution (n = 16), suggesting that HIV infection or its treatment may be involved in the pathogenesis of lipoatrophy. Vigouroux et al23 similarly noted a relationship between TNF- and fat redistribution in 131 patients. These patients showed markedly elevated levels of TNF- and its soluble receptor, sTNFR1. In a subset analysis of patients with lipohypertrophy, lipoatrophy, and mixed syndrome, sTNFR1 was significantly elevated in patients with lipoatrophy (P < 0.05) and mixed syndrome (P < 0.05) but not in patients with lipohypertrophy. Similarly, in the study by Mynarcik et al24 of sTNFR2, levels of sTNFR2 were significantly higher in HIV-infected patients with lipodystrophy than in HIV-infected patients without lipodystrophy. The elevation of TNF- in HIV fat distribution and its proven role in adipocyte apoptosis suggest a role in fat depletion. A genetic case-control study conducted in HIV-positive patients both with (n = 61) and without (n = 35) lipodystrophy found a significant difference between groups in the frequency of polymorphism -238 in the promoter region of the TNF- gene (P = 0.01), suggesting that the -238 polymorphism is a determinant in the development of HIV-related lipodystrophy.25
The enzyme 11--HSD1 has also been implicated as potentially playing a role in fat redistribution syndromes. This enzyme helps catalyze the conversion of the hormonally inactive cortisone to cortisol, which is required for adipocyte differentiation. It is expressed to a higher degree in visceral fat than in subcutaneous fat and is elevated in the presence of cortisol. The differential expression of 11--HSD1 in visceral fat, its association with cortisol, and the well-established prevalence of elevated cortisol levels in HIV suggest that this enzyme may play a role in the pathogenesis of central fat accumulation in HIV.26 To date, no specific inhibitors of 11--HSD1 exist, making it an attractive drug target.
Mitochondrial DNA toxicity via inhibition of DNA polymerase is associated with nucleoside analogues and has been hypothesized to be pathogenic in lactic acidosis, hepatic steatosis, myopathy, cardiomyopathy, peripheral neuropathy, and pancreatitis,27 and its role in fat redistribution is yet to be established. Some investigators28,29 have noted that fat redistribution in HIV shares substantial similarities with other syndromes (multiple symmetric lipomatosis) that are associated with mitochondrial DNA dysfunction. It is theorized that multiple symmetric lipomatosis is characterized by a point mutation at nucleotide position 8344 or by multiple or single mitochondrial DNA deletions.2 Cote et al27 have noted differences in the ratio of mitochondrial to nuclear DNA in non-HIV-infected controls (1.28 ± 0.38), asymptomatic HIV-infected subjects who had never received antiretroviral therapy (0.72 ± 0.19), and HIV-infected subjects with symptomatic mitochondrial toxicity while on therapy (0.28 ± 0.06). The latter value was significantly different (P < 0.001) from that seen in the control group or therapy-naive group, suggesting mitochondrial DNA toxicity is multifactorial, with both a disease and drug component.
Five recent studies have lent more weight to the hypothesis that toxicity to mitochondrial DNA may contribute to fat redistribution.30-34 Shikuma et al31 assessed the mitochondrial DNA content in subcutaneous fat tissue from the neck, abdomen, and thigh. Nineteen of 23 patients (82.6%) with lipoatrophy and a history of antiretroviral therapy for >6 months showed evidence of mitochondrial DNA depletion, compared with 2 of 20 (10%), 0 of 15, and 0 of 20 specimens from HIV patients treated with antiretroviral therapy but with no evidence of fat loss, from HIV patients who were antiretroviral naive, and from HIV seronegative patients, respectively (P < 0.001 for all comparisons). No mitochondrial DNA deletions or additions were noted in lipoatrophy, suggesting that a mitochondrial dysfunction is the result of reduced DNA content rather than DNA deletions. Similarly, Walker et al32 found that mean mitochondrial DNA content was 44% lower in buttocks fat biopsies from NRTI-treated patients (n = 19) than in the no-NRTI group (n = 5; P = 0.01), and patients with lipoatrophy (n = 11) had a 39% lower mitochondrial DNA content than patients without lipoatrophy (n = 13; P = 0.02). No mitochondrial DNA point mutations or deletions were observed. Shiramizu et al33 quantified mitochondrial DNA content per adipocyte in HIV patients who were antiretroviral naive or experienced, with or without lipoatrophy. As with the other studies, mitochondrial depletion was greatest in patients with lipoatrophy (50% depletion relative to seronegative adults), moderate in antiretroviral-experienced patients who did not have lipoatrophy (40% depletion), and least in antiretroviral-naive patients (16% depletion). Van der Valk et al34 have recently reported similar findings. In 28 patients treated with a d4T-containing regimen (n = 17) or AZT-containing regimen (n = 11), lipodystrophy occurred in 15 patients (14 of 17 [82%] in the d4T arm, and 1 of 11 [9%] in the AZT arm; P = 0.0001). Lower mitochondrial DNA content per adipocyte from subcutaneous thigh biopsies was correlated with severity of lipodystrophy (P = 0.007). Thompson et al30 have shown an increase of mitochondrial DNA (copies per adipocyte) 48 weeks after switching from a d4T-containing regimen to abacavir- or AZT-based therapy along with some improvement in arm, leg, and trunk fat. The latter 3 studies are significant, because each measured mitochondrial DNA on the cellular level (per adipocyte), thus demonstrating a cellular effect rather than a tissue effect.30,33,34

|
|
| |
| |
|
|
|