 |
 |
 |
| |
Four Drugs Versus Three-and a Four-Nucleoside Combo
|
| |
| |
Written for NATAP: A report from the 45th Interscience Conference on Antimicrobial Agents and Chemotherapy, Dec 2005
Mark Mascolini
Quadruple regimens-including nucleoside-only foursomes-grabbed the spotlight in the antiretroviral strategy arena at the 45th ICAAC. The most important news, 144-week results from AIDS Clinical Trials Group (ACTG) study 5095, confirmed several earlier studies showing that first-line quadruple regimens are no better than first-line triple therapies-if all the drugs used are strong. Two studies of tenofovir plus Trizivir (AZT, 3TC, and abacavir) in treatment-naive or treatment-experienced people held some surprises.
Efavirenz/AZT/3TC Matches Efavirenz/Trizivir
Two years before the 45th ICAAC, ACTG 5095 made a vital contribution to antiretroviral management by finding that three nucleoside reverse transcriptase inhibitors-packaged in one pill as Trizivir-did not slow HIV as well as two efavirenz-based regimens in previously untreated people [1]. The ACTG team shut down the Trizivir arm in a wink but continued to track people taking efavirenz plus AZT/3TC (as Combivir) or efavirenz plus Trizivir for a median of 144 weeks.
At that point, almost 3 years after randomization, Cornell University's Roy Gulick and ACTG colleagues could not sift out a single result suggesting a four-drug efavirenz regimen does better than a three-drug efavirenz regimen [2]. That result confirms a host of earlier studies that found no good reason to start antiretroviral therapy with four drugs instead of three [3-6].
The comparison of the two efavirenz regimens involved 765 people who started therapy with an average viral load of 72,444 copies/mL (57% were below 100,000 copies/mL) and an average CD4 count of 240 cells/mm3. Looking at the primary endpoint-two consecutive viral loads above 200 copies/mL after at least 16 weeks of treatment-Gulick counted 99 failures among 382 people (26%) taking triple therapy and 94 out of 383 (25%) taking quadruple therapy, nowhere close to a statistically significant difference (P = 0.73).
Nor did the three-drug and four-drug groups differ in time to virologic response, time to virologic failure in people with a pretreatment load above 100,000 copies/mL, percentage reaching a load below 50 copies/mL (80% to 85% in each group after 144 weeks), CD4-cell gains, treatment discontinuations, or side effect rates.
Multivariate analysis to track down factors affecting virologic failure found two variables that made failure more likely:
- Coinfection with hepatitis C virus (HCV) raised the failure risk 57% (hazard ratio 1.57, 95% confidence interval 1.02 to 2.40, P = 0.04)
- Being black instead of white raised the risk 67% (hazard ratio 1.67, 95% confidence interval 1.19 to 2.35, P = 0.003)
The first finding confirms that HIV infection can be more difficult to manage in people also battling HCV. But the racial difference is hard to explain. Gulick stressed that blacks did not differ from whites or Hispanics in overall adherence. About 85% in each ethnic group reported never missing a dose in the 4 days before each self-report.
But poor adherence among blacks at a single point-12 weeks after treatment began-did correlate with virologic failure when compared with blacks reporting good adherence at that time and with whites or Hispanics reporting good or bad adherence at 12 weeks.
Gulick speculated that genetic differences affecting efavirenz levels could play a part in this higher risk of failure. Genotyping in an earlier ACTG study found that a certain shift in a gene that codes the CYP2B6 efavirenz-metabolizing enzyme turned up more often in blacks (20%) than whites (3%) and correlated with higher efavirenz levels (P < 0.0001) [7].
Indeed, Gulick found that blacks in his study had a significantly shorter time to grade 3 or 4 side effects and a shorter time to switching from their first regimen. But ACTG gene analysts will have to look at blood samples from study participants to see if blacks had the critical CYP2B6 gene flips more than other groups, and if those flips correlate with higher efavirenz levels and more efavirenz-induced side effects.
Making that triple correlation-higher rate of critical gene shifts, leading to higher drug levels, leading to more side effects-is not easy. In the earlier ACTG trial, for example, blacks had a key CYP2B6 gene change more than whites, and they had higher efavirenz levels, but that gene shift did not correlate with more efavirenz toxicity [7]. An ICAAC study from London's Chelsea and Westminster Hospital and the University of Liverpool also failed to tie higher efavirenz levels in blacks to more efavirenz side effects [8].
Desmond Maitland and colleagues charted efavirenz concentrations in treatment-naive people beginning either 3TC/ddI/efavirenz or tenofovir/ddI/efavirenz in an open-label (nonblinded) trial. Adherence measured by three methods exceeded 99% in both treatment groups. The study ended early when the tenofovir regimen quickly proved virologically inferior, but the London-Liverpool team did measure 4- and 12-week efavirenz levels in 66 people, including 9 women and 13 Africans.
Efavirenz levels proved significantly higher at weeks 4 and 12 in women than in men, and in Africans than in whites. But high efavirenz concentrations did not correlate with neuropsychiatric symptoms assessed at week 12. People with efavirenz readings above 1100 ng/mL at weeks 4 and 12 were more likely to reach a viral load below 50 copies/mL at week 12, a finding confirming a minimum effective concentration of 1000 ng/mL for efavirenz.
First-line Rx with once-daily Trizivir/tenofovir
Trizivir's failure to keep pace with AZT/3TC/efavirenz as first-line therapy [1] proved the first report in a volley of bad news on up-front triple nucleosides. Some hoped adding tenofovir to Trizivir (AZT/3TC/abacavir) would yield a still-simple quadruple combo that would harness HIV and let untreated people avoid protease inhibitors and nonnucleosides.
Under Glaxo sponsorship, a covey of US clinicians tested that strategy in a nonblinded single-arm trial that signed up 123 people beginning therapy with a median viral load of about 120,000 copies/mL (range about 12,000 to 3,388,000 copies/mL) and a median CD4 count of 222 cells/mm3 (range 20 to 857 cells/mm3) [9].
Calvin Cohen from Boston's Community Research Initiative and coworkers upped the ante in this trial by giving all the drugs once daily, even though Trizivir is licensed as a twice-daily antiretroviral. People took two Trizivir pills and one tenofovir pill all at the same time. Week 48 results suggest the gamble did not pay off.
Fifty-two people (42%) dropped out before week 48, a distressingly high rate for a regimen assumed to be potent and tolerable. Twelve people (23%) had to quit because of a subpar virologic response, while 14 (27%) stopped the regimen because of side effects or clinical problems, and 13 (25%) stopped returning for visits. This last group, usually labeled "lost to follow-up," may well include people who did not like the regimen for some reason.
Abacavir hypersensitivity reactions in 8 people (6.5% of the study group) proved the most common nonvirologic reason for stopping Trizivir/tenofovir. One person had to abandon the regimen for each of the following reasons-mood swings, abnormal liver function test, cancer, positive syphilis test, nausea and vomiting, and nausea, vomiting, and headache. All told, 18 people (15%) had grade 3 or 4 "adverse events" and 14 (11%) had grade 3 or 4 lab abnormalities.
Because of the overlarge dropout rate, an intent-to-treat analysis that counts missing data as a failure figured about a 40% sub-50-copy virologic response rate after 48 weeks-a woeful number when compared with 48-week and longer responses to regimens hinged on efavirenz or lopinavir/ritonavir, for example. Among 64 people who stayed with the regimen for 48 weeks, the sub-50-copy response rate stood below 80%.
People who took Trizivir/tenofovir for 48 weeks had little change in lumbar spine bone mineral density or mitochondrial DNA in peripheral blood mononuclear cells. They gained arm, leg, and trunk fat. Total cholesterol, ominous low-density lipoprotein cholesterol, and triglycerides all improved with this quadruple regimen. But these are not the primary goals of first-line antiretroviral therapy.
Trizivir/tenofovir as a backup regimen
A chart review of 116 people who switched to twice-daily Trizivir plus once-daily tenofovir found that most of them maintained or improved virologic suppression with the quadruple combination [10]. After 3 to 301 weeks of follow-up (median 51 weeks), Brenda Dauer and colleagues at Frankfurt's J.W. Goethe University Hospital tallied 7 virologic failures (6%), 5 dropouts because of gastrointestinal upsets, 1 dropout because of liver toxicity, and 4 dropouts for other reasons including pregnancy, loss to follow-up, and "patient's choice."
Dauer scanned records of everyone in the clinic who took Trizivir plus tenofovir for at least 24 weeks after another regimen. About half of them opted for the four nukes because of virologic failure of their current meds, and about half to dispel side effects or to start a more convenient regimen.
The study group had tried of median of 7 antiretrovirals over a median 6.6 years. The median lowest-ever CD4 count measured 91 cells/mm3, but before starting Trizivir/tenofovir the median count stood at 283 cells/mm3.
Median viral load fell from 400 copies/mL just before Trizivir/tenofovir to 87 copies/mL after 24 weeks of the quadruple collation. In that time the median CD4 count climbed to 315 cells/mm3. A discontinued-equals-failure analysis determined that 70 of 116 people (60%) had a viral load below 400 copies/mL at week 24, and 40 (34%) were under the 50-copy mark. Among 60 people who had a viral load at or below 400 copies/mL before switching to Trizivir/tenofovir, 55 (92%) maintained that level of suppression.
Dauer discovered three factors that predicted failure to have a sub-400 viral load at week 24-more antiretrovirals tried, higher baseline viral load, and lower baseline CD4 count (Table).
Predictors of nonresponse (>400 copies/mL) after switch to Trizivir/tenofovir
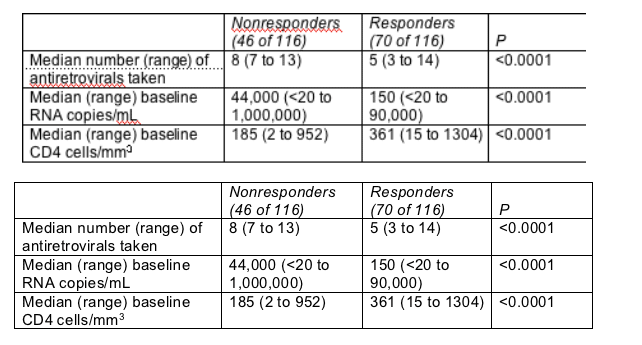
How many nucleoside resistance mutations a person had before starting Trizivir/tenofovir did not affect chances of virologic response in this cohort. Among 84 people with genotypic data on tap, the 46 with 3 or more nucleoside mutations before Trizivir/tenofovir enjoyed a median viral load drop from 10,000 to 400 copies/mL after 24 weeks of treatment.
Two thymidine analog mutations did, however, make virologic response less likely. Significantly fewer people who drove their viral load below 50 copies/mL had the T215Y/F mutation (P = 0.003) or T215Y/F plus L210W (P = 0.016).
Mark Mascolini writes about HIV infection
References
1. Gulick R, Ribaudo H, Shikuma C, et al. Triple-nucleoside regimens versus efavirenz-containing regimens for the initial treatment of HIV-1 infection. N Engl J Med 2004;350:1850-1861.
2. Gulick RM, Ribaudo HJ, Shikuma CM, et al. ACTG 5095: zidovudine/lamivudine/abacavir vs. zidovudine/lamivudine + efavirenz vs. zidovudine/lamivudine/abacavir + efavirenz for initial HIV therapy. 45th Interscience Conference on Antimicrobial Agents and Chemotherapy. December 16-29, 2005. Washington, DC. Abstract H.-416a.
3. Moyle G, Pozniak A, Opravil M, et al. The SPICE study: 48-week activity of combinations of saquinavir soft gelatin and nelfinavir with and without nucleoside analogues: Study of Protease Inhibitor Combinations in Europe. JAIDS 2000;23:128-137.
4. Katzenstein TL, Kirk O, Pedersen C, et al. The Danish protease inhibitor study: a randomized study comparing the virological efficacy of 3 protease inhibitor-containing regimens for the treatment of human immunodeficiency virus type 1 infection. J Infect Dis 2000;182:744-450.
5. Shafer RW, Smeaton LM, Robbins GK, et al. Comparison of four-drug regimens and pairs of sequential three-drug regimens as initial therapy for HIV-1 infection. N Engl J Med 2003;349:2304-2315.
6. van Leth F, Phanuphak P, Ruxrungtham K, et al. Comparison of first-line antiretroviral therapy with regimens including nevirapine, efavirenz, or both drugs, plus stavudine and lamivudine: a randomised open-label trial, the 2NN study. Lancet 2004;363:1253-1263.
7. Haas DW, Ribaudo HJ, Kim RB, et al. Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. AIDS 2004;18:2391-2400.
8. Maitland D, Boffito M, Mandalia S, et al. Correlation between therapeutic drug monitoring of efavirenz and virological response at week 12 in HIV+ subjects starting once daily antiretroviral therapy. 45th Interscience Conference on Antimicrobial Agents and Chemotherapy. December 16-29, 2005. Washington, DC. Abstract H-1902.
9. Cohen C, Elion R, DeJesus E, et al. 48 week analysis of efficacy and safety of once-daily abacavir/lamivudine/zidovudine (Trizivir) + tenofovir. 45th Interscience Conference on Antimicrobial Agents and Chemotherapy. December 16-29, 2005. Washington, DC. Abstract H-521.
10. Dauer B, Carlebach A, Stuermer M, et al. Virologic response to tenofovir DF plus Trizivir therapy in heavily ART-experienced HIV+ patients: 24 week results. 45th Interscience Conference on Antimicrobial Agents and Chemotherapy. December 16-29, 2005. Washington, DC. Abstract H-520.
|
|
| |
| |
|
 |
 |
|
|