 |
 |
 |
| |
Entecavir in HIV/HBV Coinfection - week 48 Results (ETV-038)
|
| |
| |
Reported by Jules Levin
From ICAAC, Wash DC, Dec 17, 2005
MG Pessoa (Institute De Infectologia Emilio Ribas, Sao Paula, brazil) and colleagues & BMS reported the information below & week 48 results from study 038 of coinfected patients who received entecavir. Patients were on stable 3TC containing HAART and ADDED entecavir. Previously at CROI 2005 week 24 results were reported. SUMMARY: of patients on entecavir for 48 weeks--HBV DNA reduced by mean 4.2 log at week 48. 37% of patients achieved ALT normalization. 82% achieved endpoint of HBV DNA <300 copies/ml and/or >2 log reduction from baseline. Safety profile was comparable to placebo. Entecavir had no observed negative impact on HAART efficacy parameters.
Worldwide 350-370 million people have HBV infection. Coinfection with HBV occurs in 10% of HIV+ population worldwide (HIV infection is about 40 million). People with HIV face increased liver mortality associated with coinfection. After 4 years of therapy about 90% of HBV/HIV patients develop resistance to 3TC: studies report this rate of developing resisrtance; of course, if a patient is able to maintain undetectable HIV viral load 3TC resistance should not develop, but clearly 3TC resistance does develop more often & rapidly in HIV as well as for HBV monoinfected & limits the utility of 3TC or lamivudine therapy in HBV-infected individuals. 3TC resistance also severely hampers future treatment decisions regarding HBV.
Entecavir is a potent & selective inhibitor of HBV DNA polymerase, with no significant anti-HIV activity & no inhibition of human mitochondrial polymerase; no interaction with CYP450 system or P=GP. Studies report no PK or in vitro PD interaction with 3TC, adefovir, or tenofovir. Phase III studies show superior efficacy & comparable safety to 3TC in chronic HBV, both in HBeAg+ and Ag- and in 3TC refractory patients. In phase III study in HBeAg+, HBV DNA reduction was a median of 7 logs.
The purpose of this study was to test the superiority of entecavir over placebo (PBO) when added to 3TC in coinfected patients who had incomplete HBV response to 3TC. 60 subjects were randomized 2:1 to ETV:PBO, which provides 85% power to demonstrate superiority in mean change in serum HBV DNA (by PCR) at week 24 compared with baseline.
During the first 24 weeks of this study patients were randomized during double-blind phase to ETV 1.0 mg qd (n=51) or placebo (n=17). 3Tc was continued (150 mg bid or 300 mg qd) as part of HAAER. At week 24 the open-label phase started where placebo patients received 1.0mg ETV qd (n=17) and ETV recipients continued receiving ETV (n=48).
Key Inclusion Criteria
HBV DNA of >100,000 log copies/ml by PCR.
HIV RNA <400 c/ml by PCR.
Compensated ;iver disease.
ALT <10 x ULN.
No concomitant medications with activity against HBV (except for 3TC).
No evidence of HCV or HDV infection.
Study Endpoints
Primary: mean reduction in HBV DNA (COBAS Amplicor PCR; LOQ=300 copies/ml) at week 24.
Secondary:
--mean reduction in HBV DNA (log10 copies/ml) at week 48
--HBV DNA below LOQ and/or >2 log reduction from baseline
--ALT normalization
--proportion who discontinued study drug due to AEs
--proportion with HIV virologic rebound (increase in HIV RNA from <400 to >1000 c/ml by PCR)
--mean change in CD4 count from baseline.
Patient demographics & Characteristics
94-100% men.
41 yrs old.
80-100% white, 20% Black/ African-American. 49% from South America. 35% from Europe. 15% from USA.
HBV DNA 9.13 log copies at baseline.
ALT (U/L), meam. 74 in ETV arm, 61 in PBO arm.
HBV 3TC mutation present (L180M and M204I/V; YMDD): 46/48 (96%) in ETV arm; 14/15 (93%) in PBO arm.
HIV RNA, mean: 2.15 log in ETV arm; 2.03 in PBO arm.
CD4 count, mean: 508 in ETV arm; 520 in PBO.
HBV genotype: about 60% genotype A in both arms; 20% other/missing; 12-18% AG; 6% genotype D.
MEAN HBV DNA through WEEK 48
-3.66 log copies viral load reduction at week 24 for patients receiving ETV.
Week 48: these patients had -4.20 log viral load reduction.
For patients who switched at week 24 from PBO to ETV, viral load reduction at wek 48 was -3.56 log.
Proportion of Patients with HBV DN <300 copies/ml and/or >2 log Reduction (baseline to week 48)
At week 24, patients receiving ETV: 84% (43/51) had <300 c/ml or >2 log reduction. At week 48 82% (42/51) of these patients reached the endpoint.
88% of the patients (n=15) who switched at week 24 to ETV reached the endpoint at week 48.
Proportion of Patients with ALT Normalization at Week 24 & Week 48
WEEK 24
ETV: 34% (12/35)
PBO: 8% (1/12)
WEEK 48
ETV: 37% (13/35)
PBO switch to ETV: 46%
HIV PARAMETERS Through WEEK 48
No patients experienced HIV RNA >1000 c/ml at week 24 or week 48 on ETV or PBO. Mean CD4 counts remained the same (550 in ETV & PBO arms at week 24 & 485 at week 48 in PBO arm & 567 in ETV arm. Mean HIV RNA also remained the same: in ETV arm 2.15 log at baseline, 2,09 at week 24, and 2.11 at week 48. In PBO arm, 2.03 at baseline, 2.02 at week 24, and 2.05 at week 48.
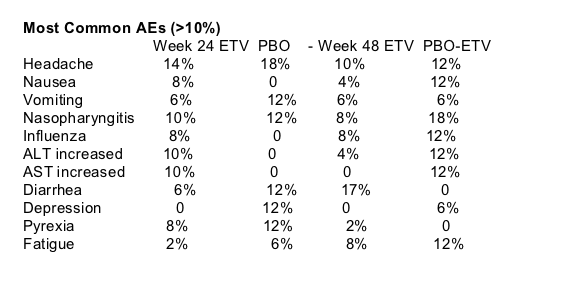
Reported Adverse Events
ON BLINDED TREATMENT
86% (n=44) of patients receiving ETV+HAART reported any AE; 0 discontinued due to AE.
Patients receiving PBO+HAART discontinued due to AE. 82% (n=14) reported any AE.
OPEN-LABEL PHASE
ETV+HAART: 0 discontinued due to AER. 81% (n=39) reported any AE.
Switch from PBO to ETV: 0 discontinued due to AE. 82% (14) reported any AE.
|
|
| |
| |
|
 |
 |
|
|