 |
 |
 |
| |
Non-AIDS "Events," Lipids, and Crusty Coronary Arteries
|
| |
| |
Written for NATAP: A report from the 45th Interscience Conference on Antimicrobial Agents and Chemotherapy
Mark Mascolini
Complications of antiretroviral therapy and non-AIDS clinical setbacks have replaced AIDS as major causes of sickness and death in people with HIV, as an ICAAC study of a large French cohort confirmed.
High lipids and heart disease rank among the most worrisome of these non-AIDS problems. Useful ICAAC studies found that tenofovir may be easier on lipids than abacavir, that lipid goals remain elusive in people taking antiretrovirals, and that the anticholesterol agent ezetimibe deserves closer scrutiny in HIV populations.
Non-AIDS complications after 5 years of HAART
Severe non-AIDS "events"-ranging from bacterial infection to cardiovascular disease-proved more common after 5 years of potent antiretroviral therapy (HAART) than a new AIDS diagnosis or death in the French APROCO-COPILOTE cohort [1]. These findings reported by Vincent Le Moing from Monpellier University Hospital confirm results from other cohorts in which liver disease, heart disease, and other clinical complications now outrank death and new AIDS diagnoses among people taking antiretroviral therapy. As Le Moing and colleagues observe, though, these non-AIDS morbidities can't always be blamed on antiretrovirals. Rather, their frequency may increase precisely because antiretrovirals let people live longer with HIV, giving other problems a chance to crop up.
The APROCO analysis involved 1281 people who started a protease inhibitor (PI) regimen from 1997 through 1999. Three quarters of the cohort is male, 25% have hepatitis C virus (HCV) coinfection, and 7% carry hepatitis B virus (HBV). When these people began a PI combo, they had a median viral load of 4.4 log (about 25,000) copies/mL and a median CD4 count of 275 cells/mm3.
After 5 years of follow-up 101 people died to yield a death rate of 1.9 per 100 person-years. Another 120 people added a new AIDS diagnosis to their charts. In contrast, 431 people had a "severe adverse event," as defined in EMEA guidelines, and 676 had an "other severe event." Incidence of severe adverse events measured 8.3 per 100 person-years and of other severe events 13.0 per 100 person-years.
AIDS remained the top cause of death in this cohort, but combined non-AIDS causes easily outnumbered AIDS-related deaths:
- AIDS: 35%
- Non-AIDS cancer not related to HBV or HCV: 8%
- Non-AIDS infection: 7%
- Accident: 7%
- Cardiovascular disease: 5%
- Intoxication or overdose: 5%
- Pancreatitis: 4%
- Suicide: 4%
- Sudden unexplained death: 2%
- Iatrogenic (physician-caused) death: 2%
- Unknown cause: 6%
Figured as incidence per 100 person-years, the top five non-AIDS killers were bacterial infection (3.2 per 100 person-years), liver disease (2.7 per 100 person-years), cardiovascular disease (1.5 per 100 person-years), suicide (1.0 per 100 person-years), and non-AIDS cancers (0.5 per 100 person-years).
Severe clinical complications outnumbered new AIDS diagnoses or death in all CD4 brackets except counts below 50 cells/mm3 (Table). Such maladies proved much more frequent than AIDS or death among both injecting drug users (IDUs) and non-IDUs, and among people with or without HBV or HCV coinfection (Table). But rates of severe clinical problems proved higher in IDUs than in non-IDUs, and in coinfected people than in people without HBV or HCV.
Cumulative 5-year probability of AIDS or death and severe "events" in APROCO
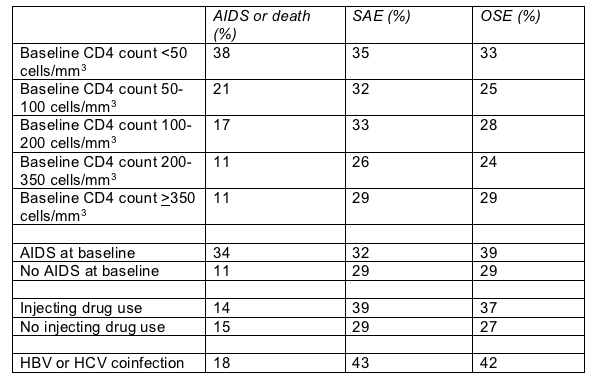

Research links antiretroviral therapy, especially with ddI plus or minus d4T, to pancreatitis [2]. And the multicohort D:A:D study sees a higher risk of myocardial infarction with longer use of antiretrovirals [3]. But cohort studies resoundingly confirm a favorable benefit-to-risk ratio for antiretroviral therapy in people with hepatitis [4,5]. And other frequent non-AIDS killers and complications in APROCO can rarely be tied to antiretrovirals.
Lipid changes after 48 weeks of abacavir or tenofovir
PIs usually get the blame for high lipids in people taking antiretrovirals, and they deserve a lot of it. But 48-week lipid trends in the RAVE trial show that the nucleoside someone takes-and the nucleoside someone may switch to-also affect cholesterol and triglyceride [6].
Graeme Moyle and colleagues at London's Chelsea and Westminster Hospital reported before ICAAC that swapping d4T or AZT for tenofovir or abacavir restores limb fat in people with moderate to severe lipoatrophy. People switching to tenofovir added 393 g of limb fat over 48 weeks, and people switching to abacavir packed on 316 g, a nonsignificant difference (P = 0.97). Study participants gained more fat if they stopped d4T (527 g with tenofovir and 357 g with abacavir) than if they dropped AZT (66 g with tenofovir and 210 g with abacavir).
At ICAAC Moyle looked at how lipids changed during 48 weeks in these 105 people, most of them white men. When people entered the study, those randomized to tenofovir and those randomized to abacavir had equivalent lipid profiles. After 48 weeks, total cholesterol, ominous low-density lipoprotein (LDL) cholesterol, and triglycerides all fell among people switching to tenofovir while rising in the abacavir group (Table). The lipid differences between the tenofovir and abacavir groups were significant for total cholesterol and LDL cholesterol. Lactates dropped with both tenofovir and abacavir.
Lipid and lactate changes 48 weeks after switching from d4T or AZT to tenofovir or abacavir
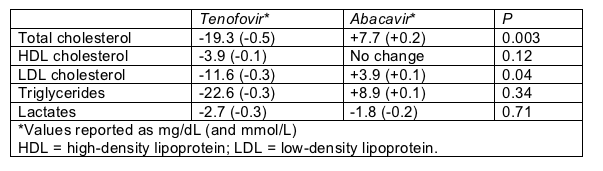
Despite the greater lipid improvements with tenofovir, Moyle did not find significant differences between study arms in proportions with high 48-week lipids as defined by the National Cholesterol Education Program. A bigger proportion randomized to tenofovir had high total cholesterol (above 240 mg/dL or 6.2 mmol/L) when the study began (38% versus 28% in the abacavir arm). And a lower proportion taking tenofovir had total cholesterol readings that high 48 weeks later (25% versus 39% in the abacavir group). But that difference in proportions with high cholesterol fell well short of statistical significance (P = 0.24).
One person in the tenofovir group started a lipid-lowering drug during the trial, compared with 8 in the abacavir group. Moyle did not report between-group differences in proportions taking PIs or nonnucleosides, and he did not report the second nucleoside in these peoples' regimens.
Lipid goals elusive in people taking antiretrovirals
Abnormal lipid levels can come back in line during antiretroviral therapy, as the just-reviewed tenofovir-versus-abacavir switch study demonstrates [6]. But that trial also shows that lipids remain out of whack in distressing proportions of antiretroviral-treated people, despite apparently appropriate antiretroviral switches and prescription of lipid-lowering drugs. A retrospective case-control comparison of US veterans with or without HIV infection verified the apparently blunted impact of antilipid drugs in people with HIV, though the study has clear limitations [7].
Stephanie Hollowell and colleagues at the Durham (North Carolina) Veterans Affairs Medical Center thumbed through records of 53 HIV-infected men who had a total cholesterol or triglyceride level above 200 mg/dL while taking antiretrovirals and then took a lipid-lowering drug for at least 2 months. The VA team matched them by age to 53 people (48 of them men) without HIV who also started an antilipid drug in the same period, 1998 through 2004. Age in both groups averaged 47.5 years-on the older side even for an HIV cohort-and 26 (49%) in each group were white.
The groups matched fairly well in baseline total cholesterol (average 275 mg/dL with HIV and 249 mg/dL without HIV), HDL cholesterol (37 and 41 mg/dL), and LDL cholesterol (164 and 169 mg/dL). But average triglyceride tallies ran substantially higher in the HIV group (average 516 mg/dL) than in the non-HIV group (average 193 mg/dL).
Veterans with HIV had higher rates of established coronary artery disease (24% versus 8%), peripheral artery disease (15% versus 0%), abdominal aortic aneurysm (7.5% versus 0%), diabetes mellitus (30% versus 11%), and family history of heart disease (36% versus 11%). Slightly more men with HIV smoked (41% versus 34%). All told, the HIV group had a substantially worse heart disease outlook than their counterparts without HIV.
The groups also differed in which antilipid agents they tried. Reflecting its relatively clean interaction record with antiretrovirals, pravastatin got prescribed for 55% of men with HIV but for no one without HIV. The preferred statin in the non-HIV group was simvastatin, taken by 85%. But because simvastatin cannot be used with PIs, only 23% in the HIV group took that drug. A smattering of men in each group took atorvastatin (1 with HIV), lovastatin (1 without HIV), fluvastatin (1 without HIV), fibrates (7 with and 4 without HIV), niacin (2 with HIV), fish oil (2 with HIV), and bile acid resins (2 without HIV).
Six months after these groups began taking antilipid drugs, 60% of the non-HIV group versus 28% of the HIV group achieved the National Cholesterol Education Program (NCEP) cholesterol target of less than 200 mg/dL, a significant difference (P = 0.0136). The HIV group had at least two disadvantages in this contest-their slightly higher baseline cholesterol readings, and their predominant reliance on pravastatin, which experts believe lacks the punch of other statins. After seeing Hollowell's cholesterol results, the Durham VA Medical Center mandated fluvastatin for people taking PIs.
Fewer people with HIV (45%) than without HIV (58%) reached the NCEP goal for LDL cholesterol, but that difference lacked statistical significance.
Significantly fewer people in the HIV group (25%) than in the non-HIV group (64%) reached the NCEP triglyceride target of less than 200 mg/dL in 6 months (P = 0.021). But this is not a big surprise since pretreatment triglycerides were so much higher in the men with HIV, and since only 11 of them (21%) took a drug that cuts triglycerides-fibrates, niacin, or fish oil.
Hollowell and colleagues underlined other limitations of their retrospective analysis-lack of randomization, samples sometimes collected without fasting, and unrecorded adherence to antilipid therapy. Still, these findings do reflect results of a randomized AIDS Clinical Trials Group study that counted few people reaching a combined NCEP target with pravastatin, fenofibrate, or both [8]. That trial did chart general improvements in LDL cholesterol, HDL cholesterol, and triglycerides with these agents.
Adding ezetimibe to push down lipids
One agent not prescribed in the just-discussed VA study is ezetimibe, a selective inhibitor of cholesterol absorption in the gut. A 24-week study of ezetimibe added to pravastatin in antiretroviral-treated people with poorly controlled lipids suggested a good response after 6 weeks, but then apparent plateaus or even rebounds in cholesterol quotients [9].
Eugenia Negredo and coworkers at Barcelona's Germans Trias i Pujol Hospital signed up 15 men and 7 women with an LDL cholesterol at or above 130 mg/dL while taking 20 mg of pravastatin daily plus antiretrovirals. No one had a triglyceride tally above 350 mg/dL to avoid interference in calculating LDL cholesterol. Everyone had taken their antiretroviral regimen for at least 3 months, with 10 people taking a PI and 8 a nonnucleoside.
Six weeks after they added 10 mg of ezetimibe daily to their regimens, average LDL cholesterol fell about 18%, total cholesterol about 14%, and triglycerides about 20%. But after 24 weeks of treatment average readings were only 5% below baseline for cholesterol, about 7% below for LDL cholesterol, and about 8% below for triglycerides. Most of the lipid rebounds between weeks 6 and 24 happened in people taking a PI. HDL cholesterol rose consistently throughout the trial, reaching an average about 8% above baseline at week 24.
A substudy of 6 people taking lopinavir/ritonavir and 1 person taking nevirapine found no interactions between ezetimibe and those drugs over 24 weeks.
An earlier retrospective study of 14 antiretroviral-treated people who added ezetimibe to other antilipid drugs graphed improvements in total and non-HDL cholesterol [10]. But this agent clearly needs further, larger study to define its role in controlling antiretroviral-induced lipid disorders.
Does HIV or its treatment thicken artery walls?
Everyone agrees that measuring carotid artery intima media thickness (IMT) is a reliable, painless way to gauge a person's risk of cardiovascular disease. But researchers measuring these artery walls in people with HIV don't agree on whether findings portend a higher heart risk in people with HIV.
A 36-month look at the French Aquitaine cohort of 223 people with HIV charted an IMT jump over the first 12 months of follow-up, but then a dropoff as people traded in PIs for other antiretrovirals and started lipid-lowering drugs [11]. A 12-month study of 346 French patients traced a slight but significant thickening of the common carotid artery over that time. That change correlated with older age, male gender, smoking, and especially a higher CD4 count at month 0 [12]. But this study found no links between IMT and type or duration of antiretroviral therapy.
A 145-person US study matched three groups for age, gender, ethnicity, smoking, blood pressure, and menopausal status-a group that continuously took PIs, a group that never took PIs, and a group without HIV [13]. Median IMT did not differ significantly between the PI group and the no-PI group, or between the combined HIV groups and the non-HIV group. Once again, traditional risk factors-including HDL cholesterol, age, and body mass index-had the biggest impact on coronary artery IMT.
But another US study-this one comparing 148 HIV-infected people with 63 age- and gender-matched controls without HIV-found significantly higher baseline IMT in the HIV group (0.91 versus 0.74 mm, P = 0.0001) [14]. Among people with HIV, five factors made higher IMT scores more likely-older age, higher LDL cholesterol, more cigarette smoking, Latino ethnicity, and hypertension. When these researchers added the non-HIV group to this multivariate analysis, HIV infection independently predicted coronary artery wall thickening (P = 0.001).
After 1 year of follow-up, median IMT rose 0.074 mm in the HIV group and fell 0.006 mm in the control group (P = 0.002). Three independent predictors of IMT progression emerged-older age, Latino ethnicity, and a lowest-ever CD4 count below 200 cells/mm3. The HIV group in this study had taken PIs for a median of 3.3 years, but antiretroviral variables did not influence IMT.
These researchers concluded that both immunodeficiency and traditional risk factors contribute to hardening artery walls in people with HIV. Their study may be more rigorous than the others because they measured carotid IMT at several points, not just one or two.
At ICAAC Danish researchers weighed in on this question, limiting their analysis to 25 HIV-infected people without one of the most common heart risk factors-smoking [15]. Thirteen people had a total cholesterol level at or below 5.5 mmol/L, while the other 12 had a reading at or above 6.5 mmol/L. Seven of 13 people in the low-cholesterol were taking a PI, compared with 8 of 12 in the high-cholesterol group. The two groups had been infected with HIV for similar times (average 13 years in the low-cholesterol group and 12 years in the high-cholesterol group), and they had similar nadir CD4 counts (144 and 123 cells/mm3).
Measuring IMT of the left and right common carotid arteries, Anne-Mette Lebech from Hvidovre University Hospital and coworkers found no big difference between the low-cholesterol group (mean 683 + 119 mM), the high-cholesterol group (656 + 99 mM), and 14 nonsmoking controls without HIV (657 + 99 mM). The HIV-negative control group, however, was older than the two HIV-infected groups, with mean ages of 51 years for controls, 49 years for the high-cholesterol group, and 45 years for the low-cholesterol group. Lebech did not report whether these age differences were statistically significant. As the studies reviewed above show, older age raises the likelihood of a higher IMT value. So a control group as young as the low-cholesterol HIV group may have had lower IMTs.
When Lebech combined the two HIV groups, she still found no IMT difference with the control group. Nor did IMT differ in HIV-infected people taking a PI (mean 658 + 117 mM) or not taking a PI (mean 687 + 99 mM). Duration of neither HIV infection nor antiretroviral therapy correlated with IMT.
Higher "good" HDL cholesterol did correlate with lower IMT (r = -0.5, P = 0.01). But total cholesterol, LDL cholesterol, and triglycerides did not influence carotid artery thickness in this analysis.
This study, though small and possibly imbalanced in the ages of people with and without HIV, tends to support the US study that found no IMT difference in people taking versus not taking PIs, and in people with HIV versus without HIV [13]. The one point on which all these studies agree is that non-HIV risk factors for heart disease consistently correlate with higher IMT-and high IMT foreshadows cardiovascular disease.
Mark Mascolini writes about HIV infection
References
1. Le Moing V, Chêne G, Cuzin L, et al. Morbidity during the 5 years following initiation of protease inhibitor therapy in HIV-infected patients. 45th Interscience Conference on Antimicrobial Agents and Chemotherapy. December 16-29, 2005. Washington, DC. Abstract H-516.
2. Reisler RB, Murphy RL, Redfield RR, Parker RA, et al. Incidence of pancreatitis in HIV-1-infected individuals enrolled in 20 adult AIDS clinical trials group studies: lessons learned. JAIDS 2005;39:159-166.
3. Data Collection on Adverse Events of Anti-HIV Drugs (DAD) Study Group. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med 2003;349:1993-2003.
4. Benhamou Y, Di Martino V, Bochet M, et al. Factors affecting liver fibrosis in human immunodeficiency virus and hepatitis C virus-coinfected patients: impact of protease inhibitor therapy. Hepatology 2001;34:283-287.
5. Qurishi N, Kreuzberg C, Luchters G, et al. Effect of antiretroviral therapy on liver-related mortality in patients with HIV and hepatitis C virus coinfection. Lancet 2003;362:1708-1713.
6. Moyle GM, Sabin S, Cartledge J, Reilly G. Lipid changes in a randomised 48-weeks, open label comparative study of tenofovir DF vs. abacavir as substitutes for a thymidine analog in persons with lipoatrophy: the Rave study. 45th Interscience Conference on Antimicrobial Agents and Chemotherapy. December 16-29, 2005. Washington, DC. Abstract H-340.
7. Hollowell S, Kaye KS, Townsend ML, et al. Achievement of NCEP goals is less frequent among HIV versus non-HIV infected veterans with dyslipidemia. 45th Interscience Conference on Antimicrobial Agents and Chemotherapy. December 16-29, 2005. Washington, DC. Abstract H-338.
8. Aberg JA, Zackin RA, Brobst SW, et al. A randomized trial of the efficacy and safety of fenofibrate versus pravastatin in HIV-infected subjects with lipid abnormalities: AIDS Clinical Trials Group Study 5087. AIDS Res Hum Retroviruses 2005; 21:757-767.
9. Negredo E, Rey-Joly C, Puig J, et al. Ezetimibe, a selective inhibitor of cholesterol absorption, as a new strategy for treatment of hypercholesterolemia secondary to antiretroviral therapy. 45th Interscience Conference on Antimicrobial Agents and Chemotherapy. December 16-29, 2005. Washington, DC. Abstract H-336.
10. von Lintig FC, Lee D, Patel P, et al. Effectiveness of ezetimibe on hyperlipidemia in HIV-1-infected patients. 6th International Workshop on Adverse Drug Reactions and Lipodystrophy. October 25-28, 2004. Washington, DC. Abstract 83.
11. Thiebaut R, Aurillac-Lavignolle V, Bonnet F, et al. Change in atherosclerosis progression in HIV-infected patients: ANRS Aquitaine Cohort, 1999-2004. AIDS 2005;19:729-731.
12. Mercié P, Thiebaut R, Aurillac-Lavignolle V, et al. Carotid intima-media thickness is slightly increased over time in HIV-1-infected patients. HIV Med 2005;6:380-387.
13. Currier JS, Kendall MA, Zackin R, et al. Carotid artery intima-media thickness and HIV infection: traditional risk factors overshadow impact of protease inhibitor exposure.
AIDS 2005;19:927-933.
14. Hsue PY, Lo JC, Franklin A, et al. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation 2004;109:1603-1608.
15. Lebrech A, Wiinberg N, Gerstoft J, et al. Predictors of atherosclerosis as measured by carotid intima-media thickness in non-smoking HIV patients. 45th Interscience Conference on Antimicrobial Agents and Chemotherapy. December 16-29, 2005. Washington, DC. Abstract H-347.
|
|
| |
| |
|
 |
 |
|
|