 |
 |
 |
| |
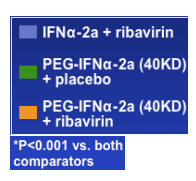
Pegasys/RBV in APRICOT Study- Adherence Improves SVR 300% in genotype 1
|
| |
| |
Reported by Jules Levin
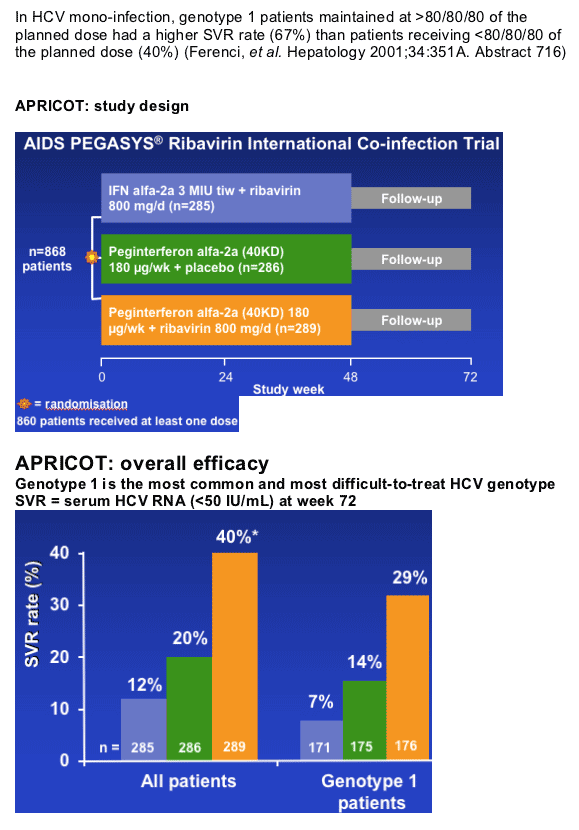
Treatment Exposure and Sustained Virological Response in Genotype 1 Patients Treated with Peginterferon alfa-2a (40KD) (PEGASYS) plus Ribavirin (COPEGUS) Therapy in APRICOT
Reported in an oral presentation at ICAAC 2005
Milos Opravil, University Hospital Zurich, Switzerland
STUDY OBJECTIVE
The objective of this analysis was to examine the effect of treatment exposure (adherence) on SVR rates in HIV-HCV genotype 1 co-infected patients treated with peginterferon alfa-2a (40KD) plus ribavirin.
AUTHOR CONCLUSIONS:
In HIV-HCV genotype 1 patients treated with peginterferon alfa-2a plus ribavirin:
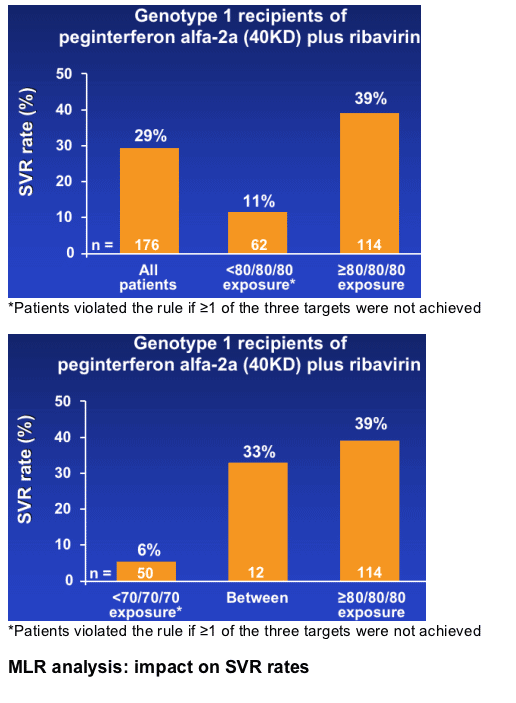
--SVR increased from 29% to 39% when patients achieved an exposure of ≥80/80/80
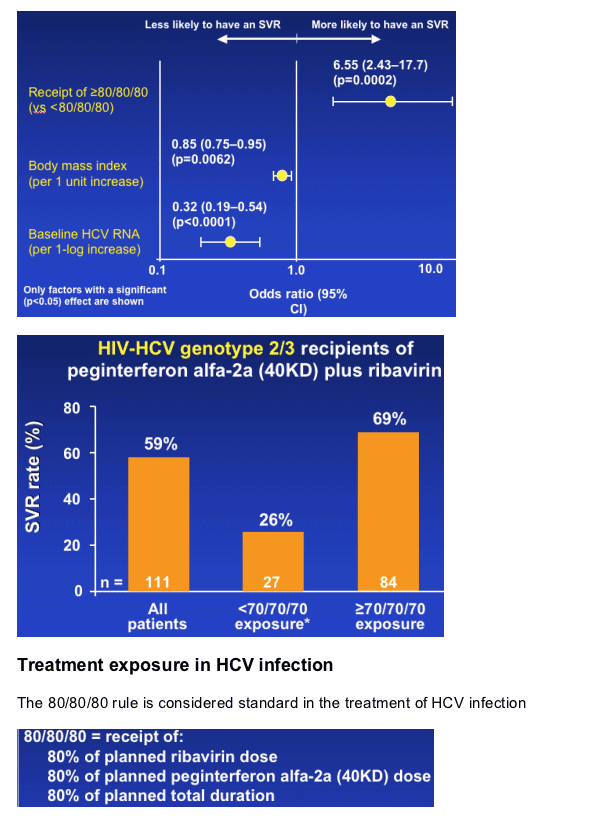
--High baseline HCV RNA and high BMI were prognostic factors for a reduced SVR rate
In HIV-HCV genotype 2/3 patients treated with peginterferon alfa-2a plus ribavirin:
--SVR increased from 59% to 69% when patients achieved an exposure of ≥70/70/70
To maximize SVR rates in HIV-HCV co-infected patients treated with peginterferon alfa-2a plus ribavirin:
--Cumulative drug doses and the duration of treatment should be ≥80% of the planned values for genotype 1 and ≥70% for genotypes 2/3
--Use of growth factors to avoid dose reductions due to hematological toxicity should be considered
--Zidovudine should be proactively switched to another NRTI before start of HCV therapy to avoid anemia
--Adherence must be actively monitored and supported
SVR rates according to exposure
Methods
Retrospective sub-analysis of 176 HCV genotype 1 patients treated with peginterferon alfa-2a (40KD) plus ribavirin
Treatment exposure assessed by multiple logistic regression (MLR) analysis
Investigation of the impact on SVR rates of:
- HCV treatment duration
- cumulative dose of each anti-HCV drug
- overall exposure (using 80/80/80 rule)
- baseline factors*
*Baseline factors considered for entry into the model:
age, gender, race, BMI, HCV RNA titer, total HAI score, qualifying ALT quotient, CD4 cell count, HAART, cirrhosis, HIV-1 RNA
Results: treatment factors
predictive of an SVR
- The relationship between various treatment factors and SVR rates were examined
- Cumulative peginterferon-alfa-2a (40KD) dose was strongly correlated with
cumulative ribavirin dose (r=0.87)
- Ribavirin dose also correlated with ribavirin treatment duration (r=0.98)
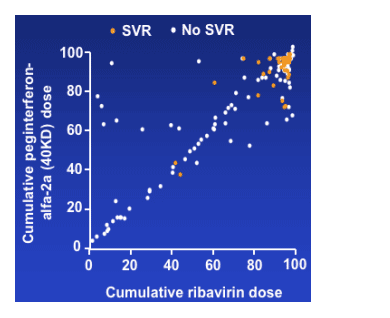
Results: treatment factors
predictive of an SVR
- Ribavirin duration (c_-score = 17.1; p<0.0001)
- Ribavirin cumulative dose (c_-score = 15.2; p<0.0001)
- Peginterferon alfa-2a (40KD) cumulative dose
(c_-score = 14.1; p=0.0002)
- Cumulative doses of both drugs were strongly correlated with treatment duration
- As very few patients with low cumulative doses had a
long duration of treatment, conclusions could not be
made for this situation
- A combined criteria (e.g. 80/80/80 rule) is therefore more appropriate
|
|
| |
| |
|
 |
 |
|
|