 |
 |
 |
| |
TMC114/r POWER 2: Adverse events & Safety profile
|
| |
| |
TMC114/r in 3-class-experienced patients: 24-week primary safety analysis of the POWER 2 study (TMC114-C202)
Reported by Jules Levin
ICAAC, Dec 17, 2005, Wash DC
D Berger,1 N Bellos,2 C Farthing,3 R Stryker,4 A Colson,5 G Pierone,6 E Lefebvre,7 M Cefalone,7 A Koester,7 K Van Baelen,8 M De Pauw8
1Northstar Medical Center, Chicago, IL; 2Southwest Infectious Disease Associates, Dallas, TX; 3AIDS Healthcare Foundation Research Center, Los Angeles, CA; 4Tower ID Medical Associates, Los Angeles, CA;
5Community Research Initiative of New England, Boston, MA; 6Treasure Chest Infectious Disease Consultants, Vero Beach, FL; 7Tibotec Inc., Yardley, PA, USA; 8Tibotec BVBA, Mechelen, Belgium
Introduction
TMC114 is a new, selective HIV PI that is highly active against a wide range of HIV isolates, both wild-type and resistant, including multidrug-resistant strains.1
This report presents the primary 24-week safety data of the POWER 2 study (TMC114-C202), one of two phase IIb, randomized, controlled, multicenter trials investigating the efficacy and safety of TMC114 with low-dose ritonavir (TMC114/r) in treatment-experienced, HIV-1–infected patients. The 24-week results of a similar trial, POWER (TMC114-C213), demonstrated that TMC114/r significantly improved efficacy outcomes compared with control PI(s)
and was generally well tolerated.2,3
The 24-week primary efficacy analysis of POWER 2 also showed that all doses of TMC114/r plus an optimized background regimen (OBR) were associated with significantly more patients achieving 31.0 log10 viral load (VL) reduction relative to baseline (p<0.01), greater VL reduction (p<0.01) and a larger increase in CD4 cell count compared with control treatment (p<0.01).4
Methods
Patients and study design
- A randomized, controlled, partially blinded, phase IIb trial to determine: the dose-response relationship of antiretroviral (ARV) activity of four different TMC114/r doses; the ARV activity of the TMC114/r dose regimens compared with control PI(s) at Week 24; and the safety and tolerability of ARV activity of TMC114/r dose regimens compared with control.
- The primary endpoint was the proportion of patients with at least a 1.0 log10 VL reduction at Week 24.
- Inclusion criteria were: male or female HIV-1–infected patients aged 318 years; treatment-experienced (prior treatment with 2 or more NRTIs for at least 3 months, with 1 or more NNRTI in a failing regimen and with 1 ormore PI for at least 3 months); on a stable PI-containing regimen, initiated at least 8 weeks prior to screening; plasma HIV-1 RNA >1,000 copies/mL; and 1 or more primary PI mutation (IAS-USA March 2003; D30N, M46I/L, G48V, I50V/L, V82A/F/T/S, I84V and L90M).5 The IASUSA list of mutations was updated while the study was ongoing; thus, the criteria were revised based on the October 2004 list.6
- During screening, investigators selected one or more control PIs and an OBR (at least two NRTIs), based on resistance testing and prior ARV history. Enfuvirtide (ENF) was allowed in the OBR. Patients were then randomized to receive either control PI(s) or one of four TMC114/r dose regimens (600/100mg bid, 400/100mg bid, 800/100mg qd or 400/100mg qd), together with the OBR.
- Prior to treatment with TMC114/r plus OBR, patients randomized to receive TMC114/r underwent a 2-week functional monotherapy period, wherein one of the four TMC114/r treatment regimens substituted for the PI(s) used at screening.
- Adverse events (AEs) and laboratory abnormalities were categorized using a modified ACTG grading severity list.7 Patients were instructed to fast for at least 8 hours prior to blood sampling for laboratory tests.
RESULTS
Patient characteristics
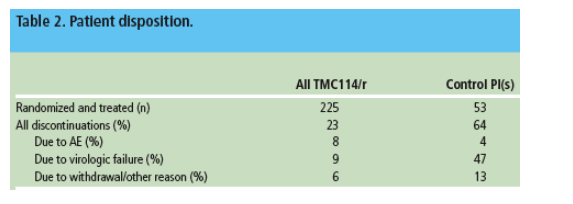
- Demographic data and baseline disease characteristics were comparable across the treatment groups (Table 1).
- The patient population in this study was more advanced in disease than those in POWER 1;2 POWER 2 patients had a longer duration of infection (13.2 vs 11.6 years), lower baseline median CD4 count (106 vs 179 cells/mm3), higher baseline VL (69% vs 59% with 320,000 copies/mL) and a higher proportion of patients had 33 primary PI mutations (66% vs 56%, IAS-USA October 2004).
PIs used in control group
- PIs selected by investigators for patients in the control group were amprenavir or fosamprenavir (42%), saquinavir (30%), lopinavir (28%), atazanavir (11%), indinavir (4%) and nelfinavir (2%). Ritonavir was used in all patients as a
pharmacokinetic enhancer.
Discontinuation and exposure
- Of the 278 patients randomized and treated, 201 patients who reached Week 24 or discontinued earlier (159 for TMC114/r and 42 for control) were included in the efficacy analysis; 77 patients who had not reached Week 24 but
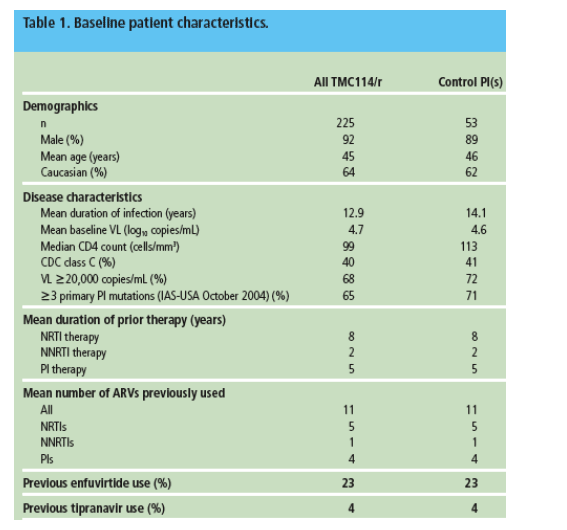
were still in the study at protocol-defined data cut-off did not contribute to the efficacy analysis. However, all 278 patients were included for the safety analysis (Table 2).
- Sixty four percent of control patients and 23% of TMC114/r patients discontinued for any reason. Among all control patients, 47% discontinued for virologic failure and 4% for AEs. Among TMC114/r patients, 9% discontinued for
virologic failure and 8% for AEs. A similar trend was observed in the 24-week analysis of POWER 1.4 No relationship between the incidence of treatment discontinuation and TMC114/r dose was observed.
- Due to the high discontinuation rate from virologic failure in the control group, these patients had a shorter mean duration of therapy (20 vs 32 weeks for TMC114/r-treated patients) and therefore had less treatment exposure. To
account for the difference in exposure, the incidence of AEs was additionally analyzed per 100 patient years of exposure.
Adverse events
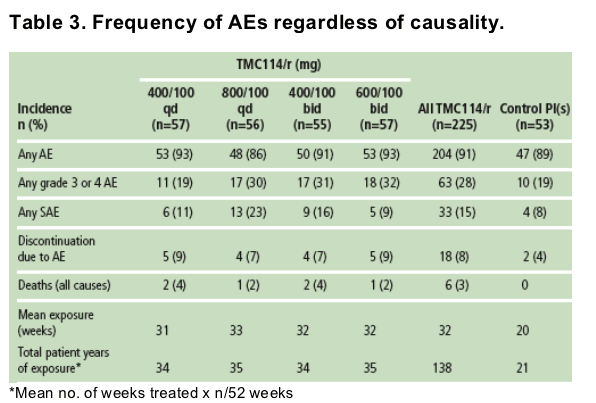
- The incidence of AEs (any grade) was comparable between TMC114/r and control patients (Table 3).
- Overall, 15% of TMC114/r and 8% of control patients reported at least one serious AE (SAE). This likely reflects the high discontinuation rate and lower treatment exposure in the control group. The most common SAE was pneumonia, reported in 2% of TMC114/r and 4% of control patients. No dose-response relationship was observed for SAE frequency.
- Six patients who received TMC114/r died. Causes of death were staphylococcal sepsis, lymphoma, lung adenocarcinoma, acute myeloid leukemia, congestive cardiomyopathy and illicit drug overdose. Investigators considered five deaths as unrelated and one as doubtfully related to study medication (lung adenocarcinoma).
- Without correcting for drug exposure, the observed incidence of commonly reported AEs was generally similar between all TMC114/r groups and control.
- The most common treatment-emergent AEs in the TMC114/r groups and occurring in at least 10% of patients were headache (17%), nausea (17%), diarrhea (16%), fatigue (16%) and insomnia (10%). Similarly, headache (17%) and diarrhea (16%) were the two most common treatment-emergent AEs observed in the TMC114/r groups in the POWER 1 trial.3 Overall, no dose-response relationship was observed for any AE.
- When exposure to treatment was corrected for, the overall incidence of AEs was lower in the TMC114/r groups than in the control arm
- thus, the incidence of headache and fatigue was substantially lower in the TMC114/r groups than in the control group (28.3 vs 43.4 events/100 patient years of exposure and 25.4 vs 48.2 events/100 patient years of exposure,
respectively)
- likewise, the incidence of diarrhea was markedly lower for TMC114/r-treated patients than in control patients (25.4 vs 57.8 events/100 patient years of exposure)
-- for nausea, no apparent difference was found between TMC114/r and the control group (28.3 vs 24.1 events/100 patient years of exposure).
- Skin rash occurred in 5% of all TMC114/r patients; no cases occurred in the control group. No dose-response relationship was observed. Almost all rash-related events were grade 1 or 2 in severity.
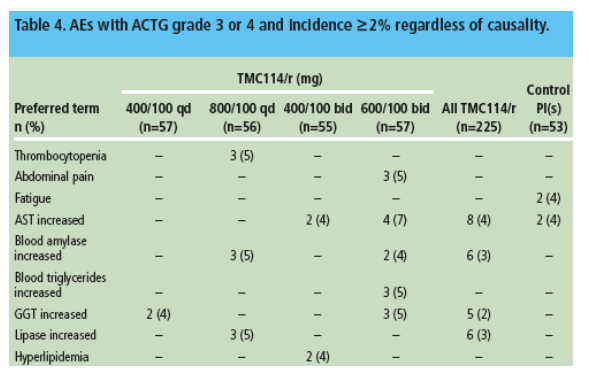
- Overall, 28% of TMC114/r and 19% of control patients reported grade 3 or 4 AEs; the difference is probably related to the high discontinuation rate and lower treatment exposure in the control group. Grade 3 or 4 AEs reported in 2%or more of TMC114/r patients consisted of increased AST (4% for both TMC114/r and control), increased blood amylase (3% vs 1.9% for control), increased GGT (2% vs none for control) and increased lipase (3% vs 1.9% for control) (Table 4).
- The overall profile of grade 3 or 4 events was comparable between TMC114/r groups. No dose-response relationship was observed.
Laboratory values
- The incidence of laboratory abnormalities was not corrected for the difference in exposure. Although some fluctuations over time were observed for some lipid, liver and hematology parameters, no dose-response relationship was observed
with TMC114/r. As with laboratory values observed in the POWER 1 trial, changes versus baseline were generally small and not considered clinically relevant.3
- Most non-graded laboratory abnormalities occurred with a similar incidence among TMC114/r and control patients. Except for grade 3 or 4 increased triglycerides, TMC114/r and control patients had a similar overall frequency for graded laboratory abnormalities.
- The majority of ALT and AST elevations were grade 1 in severity.
- Small changes were reported for vital signs and ECG parameters. Overall, QTc abnormalities were more commonly reported in the control group than TMC114/r groups.
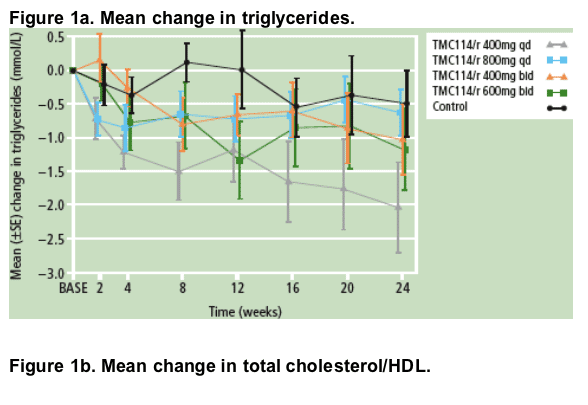
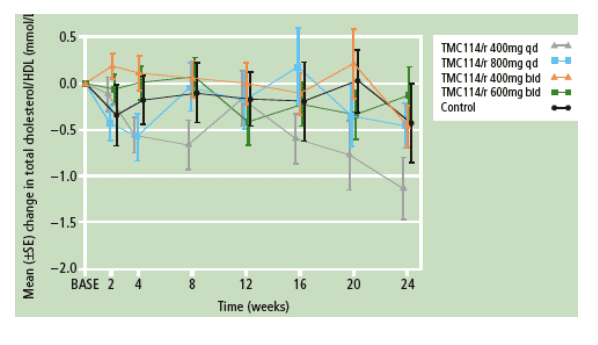
- Mean triglyceride (Figure 1a) and VLDL cholesterol levels gradually decreased over time across all groups. Total cholesterol and HDL levels remained stable over time (Figure 1b). The use of lipid-lowering agents was comparable
across all treatment groups.
|
|
| |
| |
|
 |
 |
|
|