| |
Should we treat patients with chronic hepatitis C on the waiting list?
|
| |
| |
Journal Of Hepatology
April 2005
Gregory T. Everson
University of Colorado School of Medicine, University of Colorado Health Sciences Center, 4200 East 9th Avenue, B-154, Denver, CO 80262, USA
Article Outline
- 1. The waiting list population
- 1.1. Treatment of patients with compensated cirrhosis
- 1.2. Treatment of patients with decompensated cirrhosis
- 2. Prevention of post-transplant recurrence
- 3. Management issues in treatment of decompensated cirrhosis
- 3.1. Cytopenias
- 3.2. Growth factors
- 3.3. Dose and duration of treatment
- 3.4. Post-treatment follow-up of responders
- 4. Stabilization or improvement in liver disease
- References
Abbreviations:: SVR, sustained virological response, MELD, Model for End-Stage Liver Disease, G-CSF, granulocyte-colony stimulating factor, Epo, Erythropoietin, LADR, low accelerating dose regimen, CPT, Child-Turcotte-Pugh
1. The waiting list population
The World Health Organization estimates that there are between 170 and 200 million persons worldwide who are infected with hepatitis C (HCV) 1. In the US, 1.7 million individuals have had hepatitis C for over 20 years, and by the year 2015 this number will swell to 3 million 2. If cirrhosis develops in 12.5% of individuals infected for 20 years [3-7], there will be approximately 375,000 Americans with hepatitis C and cirrhosis by the year 2015. Given current estimates of 5 million infected Europeans, there will be approximately 600,000 Europeans with hepatitis C and cirrhosis by the year 2015.
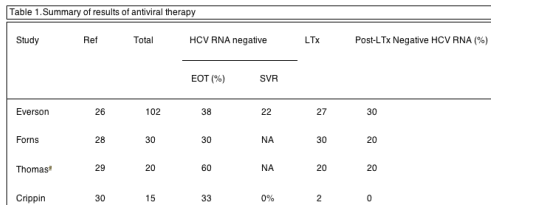
In the early stage of cirrhosis, patients are asymptomatic, lack clinical events, exhibit normal or only mild abnormalities in laboratory profile, and are defined as 'compensated'. As cirrhosis progresses, symptoms develop, laboratory tests become abnormal, the patient experiences ascites, varices, encephalopathy, spontaneous bacterial peritonitis, jaundice, or coagulopathy. The latter changes comprise the definition of 'decompensation'. Estimated rates of decompensation, development of hepatoma, and death from liver disease are 3.6-6.0%/yr, 1.4-3.3%/yr, and 2.6-4.0%/yr, respectively [8-12]. Cirrhotics with hepatitis C who experience decompensation have a five year survival of only 50% 9 and are typically listed for transplantation. Despite the need for effective therapy and the poor prognosis of decompensated cirrhotics, there have been few studies of antiviral therapy due to concern over side effects and potential complications of interferon and ribavirin (Table 1).
|
|
 |
| |
Abbreviations: Ref, reference number of citation in bibliography; EOT, end of treatment; SVR, sustained virologic response; LTx, liver transplantation. A total of 79 patients underwent liver transplantation, either deceased or living donor, and 18 remained free of HCV infection post-transplant (23%).
aAlthough there were 27 patients reported, only the 20 who received antiviral therapy are shown in this table. Seven were excluded from treatment due to platelet count ≤50,000/É l.
There is a general perception that patients with hepatitis C on the waiting list may be too sick to be treated with interferon-based therapy. However, examination of MELD (Model for End-Stage Liver Disease) scores indicates that approximately 90% of HCV patients listed at active status in the USA have MELD scores≤18. MELD score 18 corresponds to Child-Turcotte-Pugh (CTP) score of 7-9, or bilirubin 2.5mg/dL, INR 1.5, and creatinine 1.5mg./dL. The Consensus Development Conference on Liver Transplantation and Hepatitis C suggested that patients with MELD scores 18 or less could be considered for treatment 13. In addition, the AASLD practice guidelines state that patients referred for liver transplantation with mild degree of hepatic compromise could be considered for antiviral therapy, initiated at low dose, 'as long as treatment is administered by experienced clinicians, with vigilant monitoring for adverse events' 14. Thus, in contradistinction to popular belief, it is very possible that the majority of patients with chronic hepatitis C on the waiting list for liver transplantation might be candidates for antiviral therapy.
The three main goals of treatment of patients with cirrhosis on the waiting list are to:
1. achieve SVR
2. prevent post-transplant recurrence, and
3. halt disease progression.
1.1. Treatment of patients with compensated cirrhosis
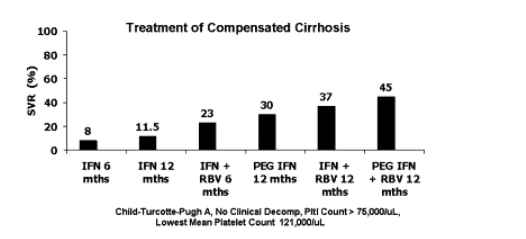
Published trials have included a small percentage of patients with either advanced bridging fibrosis or cirrhosis [15-23]. One study was restricted to patients with bridging fibrosis or compensated cirrhosis 20. Generally, virologic responses in cirrhotics were lower than responses in noncirrhotics. Nonetheless, many cirrhotics did achieve both on-treatment and sustained virologic response (SVR) (Fig. 1). The most favorable report on treatment of cirrhotics was that of Hadziyannis et al. 23, where 48 weeks of peginterferon alfa-2a plus ribavirin achieved an SVR of 50% (41% in genotype 1 and 73% in genotypes 2 and3). However, it must be emphasized that the cirrhotic patients in all of these trials were compensated and had not experienced any clinical complications of liver disease.
|
|
 |
| |
Fig. 1. Rates of sustained virologic response (SVR) with interferon-based therapy in the treatment of chronic hepatitis C in cirrhotic patients are shown. There has been progressive rise in efficacy as regimens have changed from short course of interferon monotherapy to the current standard using peginterferon plus ribavirin.
The largest experience in treatment of hepatitis C patients with cirrhosis was the Lead-In phase of the HALT C trial of retreatment of nonresponders with fibrosis 24. All patients enrolled in HALT C had prior therapy with interferon or interferon plus ribavirin. Eighty nine percent were infected with genotype 1 and 39% had cirrhosis. Entry criteria for HALT C permitted enrollment of patients with platelet counts as low as 50,000/ul; but, otherwise patients were compensated. The percentages of patients with negative HCV RNA at treatment week 20, end-of-treatment week 48, and post-treatment followup week 72 were 40, 37, and 23% in noncirrhotics, but only 26, 23, and 11% for cirrhotics. In a followup study of the entire HALT C cohort, SVR was independently and inversely related to severity of liver disease with lowest response in cirrhotics with thrombocytopenia (platelet count ≤125,000/ul) 25. The low rate of virologic response in cirrhotics in HALT C may also have been due to prior nonresponse, high proportion of patients infected with genotype 1, and protocol driven dose reductions in both peginterferon and ribavirin for cytopenias. Growth factors were not used.
The results indicate that patients with compensated cirrhosis are candidates for treatment with interferon-based therapies. However, retreatment of cirrhotics who were prior nonresponders is only marginally effective.
1.2. Treatment of patients with decompensated cirrhosis
We reported our experience with treating sicker patients many of whom had a history of clinical decompensation [26,27]. Patients were treated with the combination of interferon alfa-2b plus ribavirin using an initially low, but accelerating dose regimen (LADR). Eighty seven percent had biopsy-proven cirrhosis, and 13% had bridging fibrosis. Five percent had a pretreatment platelet count of less than 50,000/mL, and 36% dropped below 50,000/mL during treatment. Growth factors, G-CSF and erythropoeitin analogue (EPO), were used in a minority of patients. Sixty-six percent of patients had 1 or more complications before treatment, including variceal hemorrhage, ascites, spontaneous bacterial peritonitis, or encephalopathy. The mean pretreatment CTP score was 7.1+2.0. An end-of-treatment virologic response was achieved in 39% of patients (35 of 91), and SVR was achieved in 22% of patients (20 of 91). Patients who had SVR prior to transplant did not recur post-transplantation. Sixteen of the 56 nonresponders (27%) discontinued treatment because of side effects, most commonly fatigue and flu-like symptoms. Four patients experienced hepatic encephalopathy, and 3 developed infections. There were also 2 episodes of gastrointestinal hemorrhage that occurred several weeks after treatment had been discontinued. The 2 factors that predicted response to treatment were the ability to achieve target dose and duration of therapy and non-1 HCV genotype. We have extended this initial experience to include 124 patients, 90 of whom were either listed (N=43) or underwent transplantation (N=47) (Submitted, unpublished data).
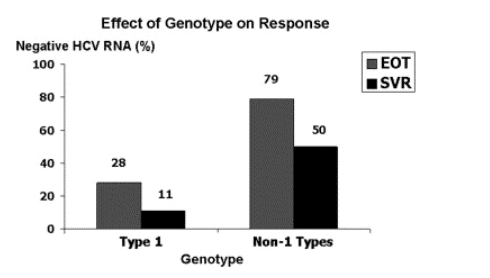
Data were particularly encouraging for patients with non-1 genotypes (mainly 2 and 3) where end-of-treatment response was 79% and SVR 50% (Fig. 2). In contrast, end-of-treatment response and SVR were 28 and 11% for patients infected with genotype 1. On the basis of these results, the authors, the Expert Panel of the ILTS consensus conference of liver transplantation, and AASLD practice guidelines have recommended consideration of patients on the waiting list for pre-transplant antiviral therapy [13,14,27]. However, the risk/benefit ratio of treating patients with decompensated cirrhosis remains to be defined by randomized controlled trials. Cirrhotic patients require close monitoring during treatment and therapy is best administered in liver clinics affiliated with liver transplant programs. Centers treating this group of patients should have extensive experience in management of advanced liver disease, hepatitis C, and liver transplant recipients.
|
|
 |
| |
Fig. 2. Virologic responses to LADR in patients with decompensated chronic hepatitis C are shown for genotype 1 versus non-1 (mainly 2 and 3) [26]. Both on-treatment and sustained virologic responses were higher with genotypes 2 and 3.
2. Prevention of post-transplant recurrence
In our initial experience, 27 patients underwent liver transplantation 26. All 19 who were HCV RNA positive prior to transplantation recurred post-transplant. In contrast, none of the 8 patients who were HCV RNA negative prior to transplant recurred post-transplant. The rate of prevention of post-transplant recurrence by pre-transplant therapy in this cohort was 30% (8/27).
Forns and al. treated 30 patients with hepatitis C and cirrhosis awaiting liver transplantation with an estimated time to transplantation of 5 months or less 28. Eighty three percent of patients were infected with genotype 1 HCV and 50% were CTP A, 43% were CTP B, and 7% were CTP C. The severity of disease in this group of patients was very similar to the group studied by Everson 26. Treatment was initiated with 3MU tiw interferon alfa-2b plus 800mg/d ribavirin but side effects were frequent and 63% required dose reductions. Nine patients (30%) achieved on-treatment clearance of HCV RNA from blood and 6 patients (20%) remained free of HCV post-transplantation. An additional 43% of nonresponders experienced a decline in viral load of ≥2 log10 prior to transplantation. This experience prompted the authors to suggest that pretransplant antiviral therapy should be considered as one of several possible strategies to prevent or reduce post-transplant HCV recurrence.
Thomas and al. studied 27 patients with chronic hepatitis C (67% genotype 1) who had undergone transplantation 29. Seven were judged to be poor candidates for interferon therapy and were not treated prior to transplantation. Twelve of the remaining 20 (60%) cleared HCV RNA with daily interferon alfa-2b monotherapy for 14+2.5 months. Four patients remained free of hepatitis C in the post-transplant period (20%), and were free of histologic hepatitis in post-transplant biopsies.
Crippin and al. treated 15 patients with severely decompensated cirrhosis awaiting liver transplantation with very low doses of interferon alfa-2b and ribavirin in 30. The conditions of the patients in this trial were more critical than in the studies by Everson 26, Forns 28, and Thomas 29 with a higher mean pretreatment CTP score (11.9+1.2). Patients in this multicenter, open-label trial were randomized to receive interferon alfa-2b 1mIU three times a week (n=3), interferon alfa-2b 3mIU three times a week (n=6), or interferon alfa-2b 1mIU once daily plus ribavirin 400mg/day (n=6). Even with low-dose interferon, 33% (5 of 15) of patients in this study experienced on-treatment HCV-RNA clearance but no patient experienced SVR (0%). It should be noted that the assay used to detect HCV RNA (branched-chain DNA assay) was less sensitive than the PCR (polymerase-chain-reaction assay) methods used in the other trials. Adverse events occurred in 13 of 15 patients (87%), and 20 of 23 events were considered severe, including thrombocytopenia (7), neutropenia (4), hepatic encephalopathy (3), hypothyroidism (1), hyperbilirubinemia (1), pancreatitis (1), staphylococcus aureus arthritis (1), ventral hernia (1), and culture-negative empyema with death (1). Because of the high rate of complications, the study was terminated, and the authors cautioned physicians regarding the hazards of antiviral therapy in patients with decompensated HCV cirrhosis.
There were a total of 79 patients in the combined experience who received antiviral therapy and underwent hepatic transplantation. Eighteen of the 79 (23%) were free of hepatitis C post-transplantation. This experience suggests that patients with decompensated cirrhosis who are candidates for liver transplantation or reside on the waiting list have significantly lower rates of SVR that patients with compensated cirrhosis (Fig. 3). Clinical status at the time of initiation of antiviral therapy appears to be the main limiting factor in decompensated HCV cirrhosis because of poor tolerability to the drug regimen [13,26-31]. Dose reductions and discontinuations will compromise clinical efficacy in the patients with decompensated cirrhosis as it does in patients with milder disease. A recent analysis suggests that dose reduction may compromise rates of SVR more in cirrhotics than noncirrhotics 25. For these reasons, it is currently recommended that patients with decompensated cirrhosis should only be treated with antiviral therapy by highly experienced clinicians 14 or in the context of a clinical trial 32.
3. Management issues in treatment of decompensated cirrhosis
3.1. Cytopenias
Cirrhotic patients are prone to neutropenia, thrombocytopenia, and anemia. Therapy with interferon, especially peginterferon, and ribavirin tends to worsen or precipitate cytopenias in this population. The benefit of higher virologic response with peginterferon, compared to nonpegylated interferon, may be counter-balanced by greater risk of cytopenias. Two strategies are used to control cytopenias: dose reduction or use of growth factors (granulocyte-colony stimulating factor, G-CSF, and erythropoietin analogues, EPO).
3.2. Growth factors
The value of either G-CSF or EPO in preventing complications or enhancing virologic response is unknown. However, the alternative strategy, dose reduction, may compromise the primary objective of achieving the highest rate of virologic response. Dietrich has demonstrated that use of EPO during treatment of chronic hepatitis C with interferon plus ribavirin can increase hemoglobin concentrations and maintain higher doses of ribavirin 33. Although we tend to favor use of growth factors over dose reduction, this strategy has not been evaluated in controlled trials. To date, there are no studies documenting the safety and efficacy of growth factors in the treatment of decompensated cirrhotic patients. For these reasons, use of growth factors to control cytopenias cannot be generally recommended.
3.3. Dose and duration of treatment
The Consensus Development Conference on Liver Transplantation and Hepatitis C and AASLD practice guidelines suggested that a low-accelerating dose regimen of therapy (LADR) is preferred in the treatment of this population [13,14]. In Forns experience, use of higher initial doses of both interferon and ribavirin resulted in dose reductions in about two-thirds of patients 28. Suggested starting doses for LADR are interferon alfa-2b, 1.5MU tiw, peginterferon alfa-2b, 0.5ug/kg/wk, or peginterferon alfa-2a, 90ug/wk, plus ribavirin, 600mg/d. Patients with creatinine clearance <50ml/min should be started at a lower dose of ribavirin, 400mg/d. Dose adjustments are made every two weeks. Interferon is first increased as tolerated to achieve full dose treatment within 2-4 weeks. Then ribavirin is subsequently increased, in increments of 200mg every two weeks, as tolerated, to attempt to achieve a target dose of 0.8 to 1.2g/d. It must be emphasized that full dose therapy is rarely achieved in patients with more severe cirrhosis due to side effects and dose-limiting cytopenias.
Complete blood count and biochemistry should be monitored every 2 weeks, until stabilization of dose, and then monthly thereafter. HCV-RNA should be measured every 3 months. Patients who fail to respond at 24 weeks of treatment with at least a 2 log drop in HCV-RNA should be dropped from treatment. Expected duration of initial treatment, once the patient achieves either target or maximum tolerated doses of both interferon and ribavirin, would be 6 months for genotypes 2 and 3, and 12 months for genotype 1, 4, 5, or 6. Relapse rates may be higher than in noncirrhotic HCV patients, particularly with genotype 1, due to inability to achieve optimal doses of both interferon or ribavirin [25-28]. Once treatment is stopped, relapse may occur. In this case it has been our practice to consider re-institution of antiviral therapy and continuation of therapy up to the time of transplantation. This decision is dependent upon the patient's virologic response, tolerance to side effects, and the estimated time to transplantation.
3.4. Post-treatment follow-up of responders
Patients should be monitored for relapse at 1, 3, and 6 months post-treatment 13, and consideration given for re-institution of treatment for those who relapse. Sustained responders may stabilize and slow or cease progression of their underlying liver disease. However, cirrhotic patients should continue to be screened for hepatocellular carcinoma, according to accepted guidelines, even after clearance of HCV. Makiyama and colleagues recently described a series of 27 patients among 1197 sustained responders to antiviral therpay who developed hepatoma, 20-90 months after successful clearance of HCV RNA 34. Only 56% of these patients had cirrhosis or advanced bridging; the remainder, 44%, had milder stages of fibrosis. Currently, it is our practice to screen cirrhotic patients who have cleared HCV RNA with alpha-fetoprotein every 6 months, and hepatic imaging (either ultrasonography or CT) annually.
Fifty percent of patients with chronic hepatitis C and cirrhosis have current or past histories of significant alcohol use or abuse. Maintenance of abstinence from alcohol must be emphasized.
4. Stabilization or improvement in liver disease
There are no published results on the efficacy of antiviral therapy in preventing need for transplantation, reversal of cirrhosis, or prevention of clinical decompensation in patients on the waiting list. In contrast, emerging data in patients with fibrosis and compensated cirrhosis suggest that interferon or interferon plus ribavirin may inhibit inflammation, stabilize fibrosis, prevent clinical deterioration, and reduce risk of hepatoma.
In Heathcote's study of compensated cirrhosis, 50% of the patients receiving 180ug/wk of pegylated interferon alfa-2a demonstrated histologic improvement on liver biopsy 20. Shiffman and colleagues demonstrated that patients who either suppressed or eradicated HCV-RNA were more likely to experience improvements in liver necroinflammation compared to virologic nonresponders 35. An analysis of 3010 patients treated in 4 randomized trials examined the impact of therapy on inflammation and fibrosis 36. Necrosis and inflammation improved in 39% of patients receiving standard interferon for 24 weeks and in 73% of patients receiving pegylated interferon alfa-2b plus ribavirin (P<0.001). Sustained viral clearance halted progression of fibrosis and reversed cirrhosis in 49% of 153 cirrhotic patients. A 2800-patient study from Japan suggested that interferon therapy was associated with a reduced risk for hepatoma, especially in patients who experienced SVR or sustained biochemical response (risk ratio, 0.516 [95% confidence interval, 0.358-0.742]; P<0.001 [37]. These results must be interpreted with caution since there is potential for selection bias in nonrandomized, retrospective analyses and responses of Western and Japanese patients to antiviral therapy may differ.
Shiffman et al. examined the effect of interferon alfa-2b (3MU tiw for 24 months) versus no treatment on histologic progression in 53 patients with prior nonresponse to interferon 38. Knodell fibrosis score decreased in treated patients from 2.5 to 1.7, and 80% had histologic improvement. In comparison, untreated patients experienced an increase in mean fibrosis scores from 2.2 to 2.4 and histologic worsening in 30%. Alric treated biochemical responders who were virologic nonresponders with maintenance interferon and also demonstrated histologic improvement 39.
Despite these encouraging results, the 'jury is still out' on the issue of interferon-based treatment in reversal of cirrhosis or prevention of disease progression. Three ongoing trials, HALT C (Hepatitis C Antiviral Long-Term treatment to prevent Cirrhosis) 40, COPILOT (Colchicine versus PEG-Intron Long-term trial) [41,42], and EPIC (Efficacy of Peg Interferon in Hepatitis C), are currently evaluating the role of maintenance therapy and may shed further light on this subject.
In summary, antiviral therapy for patients with chronic hepatitis C on the waiting list for liver transplantation is evolving. Response of cirrhotics to antiviral therapy declines with increasing severity of liver disease. HCV RNA is rendered negative during treatment in 28% of patients infected with genotype 1 and 79% of patients infected with non-1 genotypes (mainly 2 and 3). However, relapse is common and SVR is only 11% for genotype 1 and 50% for non-1 genotypes. Reasons for low SVR include high prevalence of genotype 1 HCV, inability to achieve full doses of interferon and ribavirin due to side effects and dose-limiting cytopenias, and risk of complications related to deteriorating liver function. The combined results from three reports encompassing 79 treated patients who underwent liver transplantation suggest that 23% [18/79] of post-transplant recurrence of hepatitis C can be prevented by pre-transplant antiviral therapy. Effectiveness of pre-transplant therapy will be dictated by ability to time therapy in relation to availability of donor organs. Carefully controlled trials to define safety and efficacy of antiviral therapy in decompensated cirrhosis and patients on the waiting list are desperately needed to confirm and extend these observations.
References
1. [1]World Health Organization . In: In: Weekly epidemiological record. 74:1999;.
2. [2]Armstrong GL, Alter MJ, McQuillan GM, Margolis HS. The past incidence of hepatitis C virus infection: implications for the future burden of chronic liver disease in the United States. Hepatology. 2000;31:777-782. MEDLINE | CrossRef
3. [3]Kenny-Walsh E. Clinical outcomes after hepatitis C infection from contaminated anti-D immune globulin. Irish Hepatology Research Group. N Engl J Med. 1999;340:1228-1233. MEDLINE | CrossRef
4. [4]Wiese M, Berr F, Lafrenz M, Porst H, Oesen U. Low frequency of cirrhosis in a hepatitis C (genotype 1b) single-source outbreak in germany: a 20-year multicenter study. Hepatology. 2000;32:91-96. MEDLINE | CrossRef
5. [5]Seeff LB, Buskell-Bales Z, Wright EC, Durako SJ, Alter HJ, Iber FL, et al. Long-term mortality after transfusion-associated non-A, non-B hepatitis. The National Heart, Lung, and Blood Institute Study Group. N Engl J Med. 1992;327:1906-1911. MEDLINE
6. [6]Seeff LB. Natural history of hepatitis C. Am J Med. 1999;107:10S-15S.
7. [7]Rodger AJ, Roberts S, Lanigan A, Bowden S, Brown T, Crofts N. Assessment of long-term outcomes of community-acquired hepatitis C infection in a cohort with sera stored from 1971 to 1975. Hepatology. 2000;32:582-587. MEDLINE | CrossRef
8. [8]Tong MJ, el-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med. 1995;332:1463-1466. MEDLINE | CrossRef
9. [9]Fattovich G, Giustina G, Degos F, Tremolada F, Diodati G, Almasio P, et al.. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463-472. MEDLINE
10. [10]Serfaty L, Aumaitre H, Chazouilleres O, Bonnand AM, Rosmorduc O, Poupon RE, et al.. Determinants of outcome of compensated hepatitis C virus-related cirrhosis. Hepatology. 1998;27:1435-1440. MEDLINE | CrossRef
11. [11]Hu KQ, Tong MJ. The long-term outcomes of patients with compensated hepatitis C virus-related cirrhosis and history of parenteral exposure in the United States. Hepatology. 1999;29:1311-1316. MEDLINE | CrossRef
12. [12]Khan MH, Farrell GC, Byth K, Lin R, Weltman M, George J, et al.. Which patients with hepatitis C develop liver complications?. Hepatology. 2000;31:513-520. MEDLINE | CrossRef
13. [13]Wiesner RH, Sorrell M, Villamil F. International liver transplantation society expert panel. Report of the first international liver transplantation society expert panel Consensus conference on liver transplantation and Hepatitis C. Liver Transpl. 2003;39:S1-S9.
14. [14]Strader DB, Wright T, Thomas DL, Seeff LB. AASLD practice guideline: diagnosis, management, and treatment of Hepatitis C. Hepatology. 2004;39:1147-1171. MEDLINE | CrossRef
15. [15]Davis GL, Balart LA, Schiff ER, Lindsay K, Bodenheimer HC, Perrillo RP, et al.. Treatment of chronic hepatitis C, with recombinant Interferon alfa. A multicenter randomized, controlled trial. Hepatitis interventional therapy group. N Engl J Med. 1989;321:1501-1506. MEDLINE
16. [16]Di Bisceglie AM, Martin P, Kassianides C, Lisker-Melman M, Murray L, Waggoner J, et al.. Recombinant Interferon alfa therapy for chronic hepatitis C. A randomized, double-blind, placebo-controlled trial. N Engl J Med. 1989;321:1506-1510. MEDLINE
17. [17]Hoofnagle JH, Di Bisceglie AM. The treatment of chronic viral hepatitis. N Engl J Med. 1997;336:347-356. MEDLINE | CrossRef
18. [18]McHutchison JG, Gordon SC, Schiff ER, Shiffman ML, Lee WM, Rustgi VK, et al.. Interferon alfa-2b alone or in combination with Ribavirin as initial rreatment for chronic hepatitis C. Hepatitis interventional therapy group. N Engl J Med. 1998;339:1485-1492. MEDLINE | CrossRef
19. [19]Davis GL, Esteban-Mur R, Rustgi V, Hoefs J, Gordon SC, Trepo C, et al.. Interferon alfa-2b alone or in combination with Ribavirin for the treatment of relapse of chronic hepatitis C. International hepatitis interventional therapy group. N Engl J Med. 1998;339:1493-1499. MEDLINE | CrossRef
20. [20]Heathcote EJ, Shiffman ML, Cooksley WG, Dusheiko GM, Lee SS, Balart L, et al.. Peginterferon alfa-2a in patients with chronic hepatitis C and cirrhosis. N Engl J Med. 2000;343:1673-1680. MEDLINE | CrossRef
21. [21]Fried MW, Shiffman ML, Reddy RK, Smith C, Marino G, Goncales F, et al.. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C Infection. New Engl J Med. 2002;347:975-982. CrossRef
22. [22]Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman ML, Reindollar R, et al.. Peginterferon alfa-2b plus Ribavirin compared to Interferon alfa-2b plus Ribavirin for initial treatment of chronic hepatitis C: A randomized trial. International Hepatitis Interventional Therapy Group. Lancet. 2001;358:958-965. MEDLINE
23. [23]Hadziyannis SJ, Sette H, Morgan TR, Balan V, Diago M, Marcellin P, et al.. Pegasys International Study Group. Peginterferon alfa-2a and Ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and Ribavirin dose. Ann Intern Med. 2004;140:346-355.
24. [24]Shiffman ML, Di Bisceglie AM, Lindsay KL, Morishima C, Wright EC, Everson GT, et al.. Peginterferon alfa-2a and Ribavirin in patients with chronic hepatitis C who have failed prior treatment. HALT-C Trial Group. Gastroenterology. 2004;126:1015-1023. MEDLINE
25. [25]Everson GT, Hoefs JC, Malet P. Impaired virologic response in patients with advanced liver disease due to chronic hepatitis C is independently linked to severity of disease: results from the HALT C trial. Hepatology. 2004;40:180A.
26. [26]Everson GT, Trotter JF, Kugelmas M. Long-term outcome of patients with chronic hepatitis C and decompensated liver disease treated with the LADR protocol [low-accelerating-dose-regimen]. Hepatology. 2002;36:297A.
27. [27]Everson GT. Treatment of patients with hepatitis C virus on the waiting list. Liver Transpl. 2003;9:S90-S94.
28. [28]Forns X, Garcia-Retortillo M, Serrano T, Feliu A, Suarez F, de la Mata M, et al.. Antiviral therapy of patients with decompensated cirrhosis to prevent recurrence of hepatitis C after liver transplantation. J Hepatol. 2003;39:389-396. Abstract | Full Text | PDF (772 KB) | MEDLINE | CrossRef
29. [29]Thomas RM, Brems JJ, Guzman-Hartman G, Yong S, Cavaliere P, Van Thiel DH. Infection with chronic hepatitis C virus and liver transplantation: A role for Interferon therapy before transplantation. Liver Transpl. 2003;9:905-915.
30. [30]Crippin JS, McCashland T, Terrault N, Sheiner P, Charlton MR. A pilot study of the tolerability and efficacy of antiviral therapy in hepatitis C virus-infected patients awaiting liver transplantation. Liver Transpl. 2002;8:350-355.
31. [31]Heathcote EJ. Treatment considerations in patients with hepatitis C and cirrhosis. J Clin Gastroenterol. 2003;37:395-398. MEDLINE
32. [32]Fontana RJ, Everson GT, Tuteja S, Vargas HE, Shiffman ML. Controversies in the management of hepatitis C patiens with advanced fibrosis and cirrhosis. Clin Gastroenterol Hepatol. 2004;2:183-197.
33. [33]Dietrich DT, Wasserman R, Brau N, Hassanein TI, Bini EJ, Bowers PJ, et al. Once-weekly epoetin alfa improves anemia and facilitates maintenance of ribavirin dosing in hepatitis C virus-infected patients receiving ribavirin plus interferon alfa. Am J Gastroenterol. 2003;98:2491-2499. MEDLINE | CrossRef
34. [34]Makiyama A, Itoh Y, Kasahara A, Imai Y, Kawata S, Yoshioka K, et al.. Characteristics of patients with chronic hepatitis C who develop hepatocellular carcinoma after a sustained response to interferon therapy. Cancer. 2004;101:1616-1622. MEDLINE | CrossRef
35. [35]Shiffman ML, Hofmann CM, Thompson EB, Ferreira-Gonzalez A, Contos MJ, Koshy A, et al.. Relationship between biochemical, virological and histological response during interferon treatment of chronic hepatitis C. Hepatology. 1997;26:780-785. MEDLINE | CrossRef
36. [36]Poynard T, McHutchison J, Manns M, Trepo C, Lindsay K, Goodman Z, et al.. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122:1303-1313. MEDLINE
37. [37]Yoshida H, Shiratori Y, Moriyama M, Arakawa Y, Ide T, Sata M, et al.. Interferon therapy reduces the risk for hepatocellular carcinoma: national surveillance program of cirrhotic and noncirrhotic patients with chronic hepatitis C in Japan: IHIT study group-inhibition of hepatocarcinogenesis by interferon therapy. Ann Intern Med. 1999;131:174-181. MEDLINE
38. [38]Shiffman ML, Hofmann CM, Contos MJ, Luketic VA, Sanyal AJ, Sterling RK, et al.. A randomized, controlled trial of maintenance interferon therapy for patients with chronic hepatitis C virus and persistent viremia. Gastroenterology. 1999;117:1164-1172. MEDLINE
39. [39]Alric L, Duffaut M, Selves J, Sandre K, Mularczyck M, Izopet J, et al.. Maintenance therapy with gradual reduction of the interferon dose over one year improves histological response in patients with chronic hepatitis C with biochemical response: results of a randomized trial. J Hepatol. 2001;35:272-278. Abstract | Full Text | PDF (174 KB) | MEDLINE | CrossRef
40. [40]Lee WM, Dienstag JL, Lindsay KL, Lok AS, Bonkovsky HL, Shiffman ML, et al.. The HALT C trial group. Controlled Clinical Trials. 2004;25:472-492. Abstract | Full Text | PDF (581 KB) | MEDLINE | CrossRef
41. [41]Afdhal N, Levine R, Black M. Colchicine versus PEG-Intron longterm: 1 year data from the COPILOT study (abstr). Gastroenterology. 2002;122:218.
42. [42]Afdhal N, Freilich B, Levine R, Black M, Brown R, Monsour H, et al.. Colchicine versus Peg-Intron long-term (COPILOT) trial: interim analysis of clinical outcomes at Year 2. Hepatology. 2004;40:238A.
|
|
| |
| |
|
|
|