 |
 |
 |
| |
CCR5 Inhibitor SCH417690: antiviral activity
|
| |
| |
"SCH 417690: antiviral activity of a potent new CCR5 receptor antagonist"
All 3 CCR5 inhibitors are in advanced stages of development and are a completely new class of drugs. This means that patients with drug resistance to currently available HIV drugs should respond well to these new drugs, and this will open up an entirely new approach to treatment, yet to be fully explored. This may be a new era similar in impact to when protease inhibitors became available in 1996.
Schuerman presented a talk at the Intl AIDS Society Conference about the 3rd new CCR5 inhibitor in development. He talked about the dose ranging study for this drug which is the advanced development stages now. The study was designed to evaluate safety, tolerability, pharmacokinetics, and antivial activity of SCH417690 in HIV+ individuals.
48 HIV-infected individuals were enrolled into a sequential rising dose study to evaluate 10 mg, 25 mg, and 50 mg twice daily of SCH417690 versus placebo for 14 days.
Within each of the 3 cohorts (n=16), 12 subjects received SCH417690 and 4 subjects received placebo in a randomized, blinded design.
Subjects were either treatment-na´ve or had no antiretroviral treatment for a minimum of 8 weeks prior to study enrollment, had CCR5-tropic virus only, and had CD4 cell counts >200.
All subjects were followed for an additional 14 days after completion of dosing.
RESULTS
SCH417690 was safe and well tolerated.
Analysis of the pharmacokinetic profile in the three dose levels showed dose proportionality with steady-state Cmin values above SCH 417690's IC90.
There was a statistically significant dose-related suppression of HIV RNA viral load across all 3 dose levels.
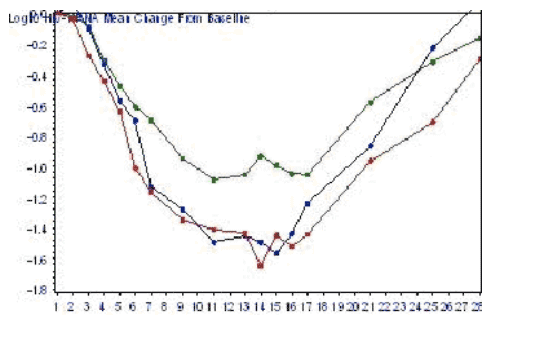
Mean log10 reductions in HIV RNA were -0.93, -1.49, and -1.62 for the 10 mg, 25 mg, and 50 mg twice daily groups, respectively.
The 25mg and 50mg twice daily doses showed a similar maximum antiviral effect that was superior to the 10mg dose.
HIV RNA slowly returned toward baseline after the completion of dosing.
CD4 counts also improved during treatment.
The authors concluded that SCH417690 demonstrated potent antiviral activity against CCD5-using HIV-1 strains at all doses studied. These findings, along with the marked post-antiviral effect which lasted a number of days after completion of dosing, support further clinical development of SCH417690. This drug also is in advanced stages of study in patients.
In red is the viral load reduction for the 50 mg dose and in blue is the 25 mg dose.
|
|
| |
|
 |
 |
|
|