 |
 |
 |
| |
24 vs 48 weeks Pegasys/RBV in HCV Genotype 2/3 Coinfection:
does this study answer the question
|
| |
| |
Reported by Jules Levin
An Italian research group (Puoti, Zanini et al, Master coinfection study group, IAS/Rio, poster MoPpLB0103) studied whether 24 or 48 weeks therapy (Pegays+ribavirin) showed better viral outcomes for HCV/HIV patients with genotype 2/3. As it turned out 92-100% of study patients had genotype 3. The incidence of relapses in patients who were HCV RNA negative at end of treatment was the main outcome compared between the two groups.
The authors concluded that these study results "suggest that the optimal duration of therapy in coinfected patients with genotypes 2/3 is at least 48 weeks", because the study observed a higher relapse rate among patients who were HCV negative after 24 weeks therapy compared to those who were HCV negative after 48 weeks therapy.
Does this study answer the question?
I don't think this study was conducted in a manner to provide a conclusive answer to the question. To better address the question therapy and patients should be stratified by baseline viral load, the presence of cirrhosis, and perhaps other characteristics. That is, if you select patients with all the characteristics that would lend themselves to achieving an SVR with only 24 weeks therapy, that would be a better patient population in which to study this question. Such characteristics would include: patients with >500 CD4s and <50 copies/ml, with reconstituted immune systems; no fatty liver; no cirrhosis, early stage HCV disease; low HCV viral load; full adherence; use of EPO; undetectable HCV RNA by week 4 or 8. Perhaps these patients could achieve better outcomes with shorter than 48 weeks therapy. Even with these considerations it is reasonable to think that in HIV at least 48 weeks therapy is necessary to optimize response rates.
BACKGROUND: In HCV monoinfection, study results show the 24 weeks therapy with peginterferon plus ribavirin may be adequate duration of therapy for patients with genotype 2/3. The Hadziyannis study of Pegasys plus 800mg daily ribavirin found 73-78% SVR rates for genotype 2/3 for 24 or 48 weeks therapy regardless of viral load levels at baseline of weight. This study set a standard for 24 weeks therapy with peginterferon plus RBV in genotypes 2/3 who are HCV monoinfected. However, this study did not examine the effects of adherence on outcomes of 24 vs 48 weeks therapy. It is possible that high adherence levels (80-100%) would have resulted in a better SVR rate with 48 weeks therapy. Dose reductions of peginterferon or ribavirin were likely to have occurred more often for patients receiving 48 weeks therapy. So, you could make a case that a patients with 100% adherence and no dose reductions would achieve better SVR rates with 48 weeks therapy. But, perhaps 24 weeks might be adequate for most patients.
A study published in the NEJM (June 2005) found that for HCV monoinfected genotype 2/3 patients with undetectable HCV RNA after 4 weeks of peginterferon alfa-2b plus ribavirin, 12 weeks of therapy was as effective as 24 weeks of therapy in terms of SVR rates. But there was a slight suggestion that the risk for relapse could be a bit higher in the 12-week group. Here is link to study article:
Peginterferon Alfa-2b and Ribavirin for 12 vs. 24 Weeks in HCV ...
www.natap.org/2005/HCV/062305_01.htm
Another consideration is long-term outcomes over the course of many years. Although SVR rates appear the same with 24 or 48 weeks duration of therapy for patients in certain categories, will this hold up 10-20 years in the future?
BACK TO ITALIAN COINFECTION STUDY
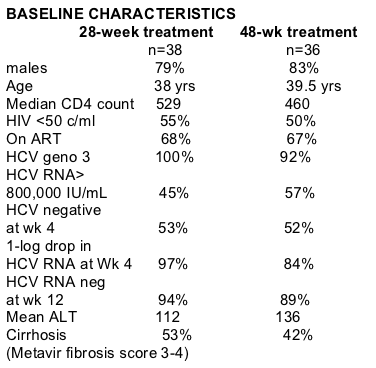
36 patients received 48 weeks of Pegasys + ribavirin therapy (800-1200 mg/daily dosed by weight). Only 2 (11%) of the 19 patients concluding the 48-week treatment period relapsed. Per-protocol analysis showed a significantly lower relapse rate in the patient group receiving 48 weeks therapy compared to those receiving only 24 weeks therapy (11% vs 40%, p=0.04), "that was not observed in the ITT analysis 53% vs 40%" (stated in the program book).
STUDY AIM: to optimize duration of treatment for chronic hepatitis C in persons infected by HIV and HCV genotype 2 or 3.
STUDY DESIGN: Randomized prospective, open, controlled trial,
METHODS: The study enrolled patients on stable ART >6 weeks, CD4 count >200 & HIV RNA <10,000 c/ml, or ART na´ve with CD4 count >300. All patients received Pegasys at 180 mcg once a week by subcutaneous injection in combination with ribavirin 10.6-13 mg/kg/day for 28 weeks. Ribavirin dose was based on weight: <65 kg, 800 mg daily; 66-85 kg, 1000 mg daily; >85 kg, 1200 mg daily.
All patients showing negative HCV RNA at 24 weeks were randomized at the 28th week to: stop treatment or continue treatment for an additional 20 weeks for a total of 48 weeks therapy. Of the128 coinfected patients who were enrolled in the study 74 were HCV PCR negative after 24 weeks of therapy.
The incidence of relapses in patients who were HCV RNA negative at end of treatment was the main outcome compared between the two groups. 128 patients were enrolled. 46 patients (36%) stopped treatment before 24 weeks: 20 (16%) because of adverse events and 26 (20%) for intolerance. 30 of these 46 (65%) showed HCV RNA negativity during treatment, but 14 out of 30 (47%) relapsed after stopping treatment. 8 out of 82 patients (10%) did not show negative HCV RNA at the end of the first 24 weeks of treatment. THEN, 74 patients were randomized: 38 to stop treatment and 36 to continue treatment for a total of 48 weeks. 15 (40%) of the patients who stopped at 24 weeks relapsed after treatment withdrawal.
17 patients from the group that continued treatment prematurely stopped therapy: 4 for serious adverse event (2 relapsed) and 13 for intolerance (11 relapsed). Only 2 (11%) of the 19 patients concluding the 48-week treatment period relapsed.
ADVERSE EVENTS
Serious adverse events were reported in 40 (31%) of the 128 patients in weeks 0-24, and 8 (22%) of patients in weeks 28-48 of treatment. The overall SAE-related treatment withdrawal rate was 18%: 12% (n=16) of the 128 patients during weeks 0-24, and 8% (n=3) in the 36 patients during weeks 28-48. This shows patients probably got used to the side effects or were better able to tolerate them after a while. Or the patients continuing therapy had more incentive to continue.
During weeks 0-24 (n=128) there were 8 grade 3 neuropsyciatric events (7/8) cases resolved with peginterferon and/or RBV dose reduction, and 5 grade 4 cases. During weeks 28-48 there was 1 grade 3 and 1 grade 4 neuropsychiatric event. The grade 3 event resolved with peginterferon and/or RBV dose reduction.
ANEMIA: There were 6 cases of grade 3 anemia (all resolved with peginterferon and/orRBV dose reduction) and 1 case of grade 4 anemia during weeks 0-24. During weeks 28-48 there were 2 cases of grade 2 anemia (resolves with peginterferon and/or RBV dose reduction) and 1 case of grade 4 anemia.
NEUTROPENIA: there were 10 cases of grade 3 neutropenia (all resoleved with peginterferon and/or RBV dose reduction) and 1 case of grade 4 during weeks 0-24. During weeks 28-48 there were 2 cases of grade 3 neuropenia (all resolved with peg and/or RBV dose reduction) and no grade 4 cases.
THROMBOCYTOPENIA: there was 1 case of grade 3 (resolved with peg and/or RBV dose reduction) and 2 cases of grade 4. There were no cases during weeks 28-48.
There was 1 case of neuropathy reported, grade 4 during weeks 28-48.
The study apparently did not use adjunctive therapy such as EPO for anemia or neutropenia.
|
|
| |
|
 |
 |
|
|