 |
 |
 |
| |
873140 Exhibits Potent Antiviral Activity Against a
Broad Panel of HIV-1 Envelopes From Treatment
Naive and Experienced Subjects
|
| |
| |
......All isolates were susceptible to the GW873140 (excluding the X4 component of the mixed tropic samples and treatment experienced patient samples appeared more susceptible to the drug.....
Reported by Jules Levin
IAS-Rio (July 2005) Poster Number TuPe6.1B13
James Demarest1, Tab Bonny1, Cindy Vavro1, Celia LaBranche1, Kathryn Kitrinos1, Charlene McDanal1, Sara Sparks1, Jeannette Whitcomb2,
Wei Huang2, Chris Petropoulos2, Stephen Piscitelli1
1GlaxoSmithKline, Research Triangle Park, NC, United States of America; 2ViroLogic, San Francisco, CA, United States of America
873140 is a novel spirodiketopiperazine CCR5 antagonist that demonstrates
in vivo (in human patients) anti-HIV activity (mean 1.66 log10 decrease at nadir) following 10 days of monotherapy (Ref 1). Previous in vitro (in the test tube) studies using PBMC (cells) infected with a limited panel of HIV clinical isolates (patient blood samples) showed low/sub-nanomolar anti-HIV activity (low level of drug amount needed to suppress HIV) for 873140 (Ref 2). The current study assessed the anti-HIV activity of 873140 when tested against a broad panel of HIV-1 envelopes from clinical samples (patient blood samples).
AUTHOR CONCLUSIONS
873140 is active against a broad array of HIV-1 envelopes from both naive
and experienced subjects from geographic regions representing different
HIV-1 clades
The CCR5-using components of R5X4-tropic envelopes were susceptible in
this assay using a CD4+ cell line that only expresses CCR5
Plasma concentrations of 873140 achieved in clinical studies exceed by
several fold the IC50 values of viruses in clinical plasma samples, suggesting
that 873140 should provide antiviral activity in HIV-1 infected individuals
AUTHOR DISCUSSION
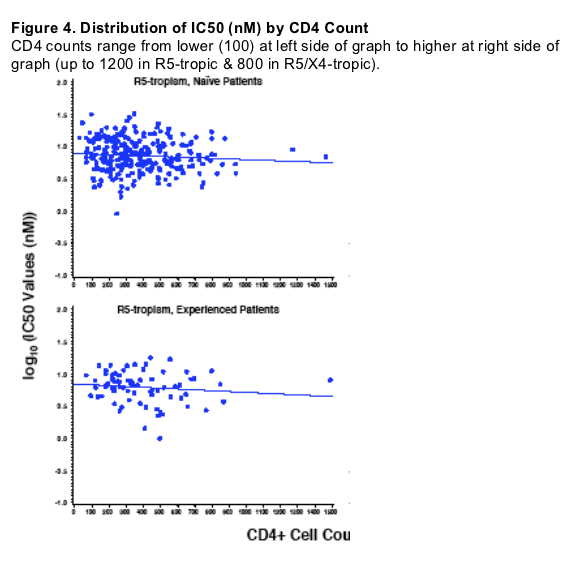
873140 is active against R5-tropic envelopes derived from both naive
and experienced patients representing a range of CD4 count and plasma
HIV-1 RNA levels
-- The IC50 range is higher than previously observed using a PBMC assay
system
- may relate to differences in CCR5 expression and/or the assay system
(pseudotype vs replication competent virus)
-- Previous studies have shown that 873140 is also active against HIV
resistant to enfuvirtide (Ref 3)
-- The reason(s) for lower IC50 values with envelopes from experienced
patients than naives are unknown
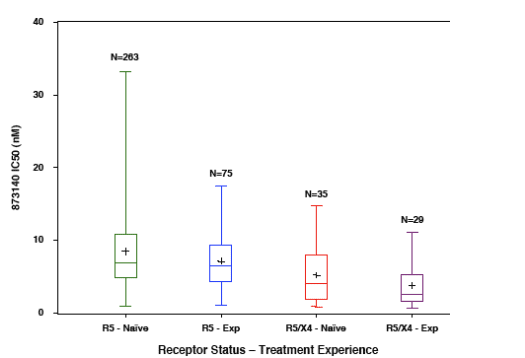
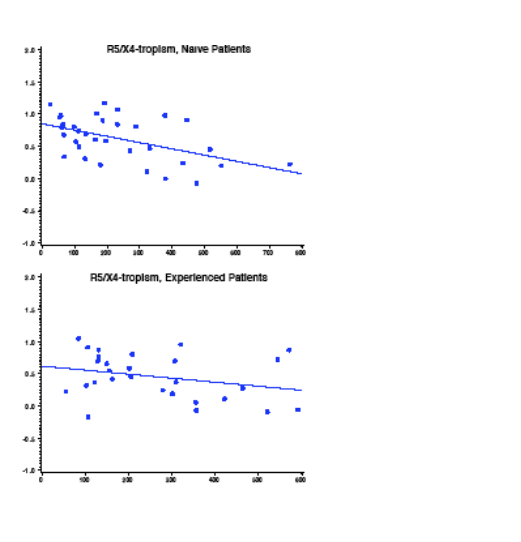
R5X4-tropic samples have lower IC50 values than R5-tropic
These samples:
-- may contain a mixture of R5-, dual-, and/or X4-tropic viruses
-- may not be sensitive to CCR5 antagonists in vitro when tested in PBMC
that express both CCR5 and CXCR4
-- are sensitive to CCR5 antagonists in assays using cells that only express
CCR5
-- The lower IC50 values may be due to the relative tropism mixture in the
R5X4 samples or to decreased efficiency of entry for R5X4-tropic
envelopes
- Previous clonal analysis showed dual-tropic envelopes were more
sensitive to 873140 than are R5-tropic Envs (Ref 4)
Tropism, treatment history, and CD4 count were associated with IC50
values
-- The CD4 count effect was more pronounced with R5X4-tropic samples
RESULTS
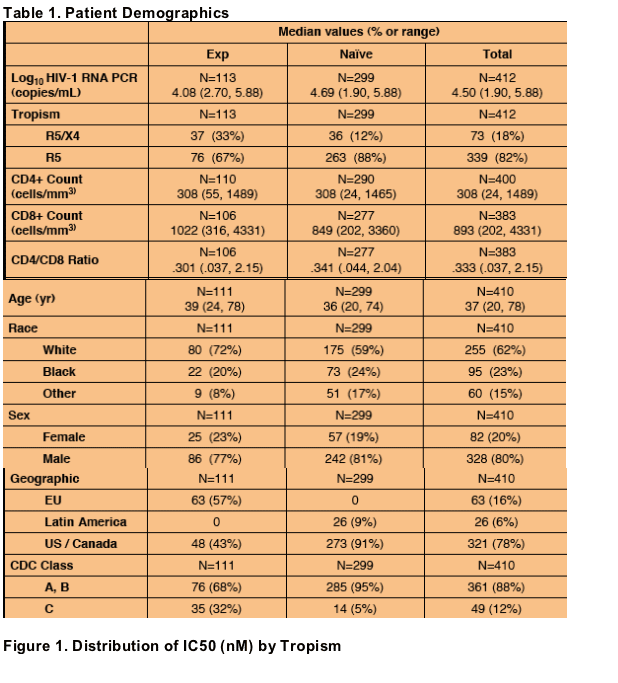
Median IC50 for R5-tropic (n=338) samples was 6.81 nM (range: 0.89-33.19).
The CCR5-using components of R5X4-tropic (n=64) samples were susceptible with a median IC50 of 3.61 nM (0.67-14.72).
95% of all samples had IC50 values < 16.5 nM.
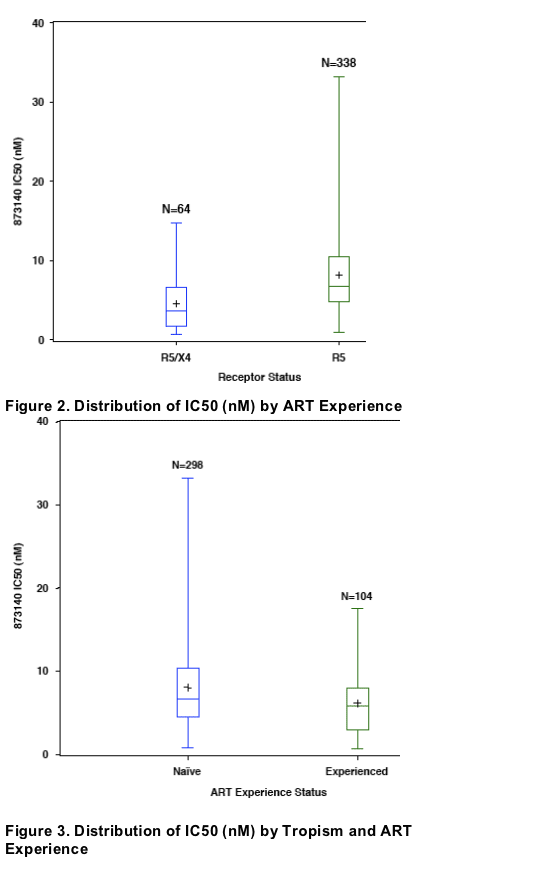
Samples from treatment experienced subjects were more susceptible to
873140 than naive (p<0.0001), with median IC50s of 5.83 nM (0.67-17.53)
vs 6.71 nM (0.84-33.19), respectively.
In the following figures, the box represents the 25th to 75th percentile, middle
line of box is median, "+" is mean, extended line represents minimum and
maximum values.
METHODS
Susceptibility (IC50 (nM)) of HIV envelopes in plasma (N=402) to 873140
was evaluated by the ViroLogic PhenoSense HIV Entry assay
_ --This assay uses an engineered cell line that expresses CD4 and only
CCR5
Samples were from treatment experienced (still on the failing regimen at
sample collection) and naive subjects (Table 1)
Used Analysis of Variance (ANOVA) and linear regression methods to
describe IC50 to 873140 in terms of baseline characteristics (n=402 with
IC50 data)
_ --IC50 values were log10 transformed in this analysis
_ --Initial predictors were examined alone to screen variables to use in model
selection
One Way ANOVA Results for Dependent Variables
Plasma HIV-1 RNA PCR (p=0.0407)
Tropism (R5 only vs R5/X4) (p<0.0001)
CD4+ Cell Count
CD8+ Cell Count
CD4/CD8 ratio
Age, ethnic origin (white vs other), gender
Geographic region (EU vs NA + Latin Am)
-- No naive patient samples were from EU
CDC Category (C versus others)
Prior antiretroviral treatment (naive vs exp) (p=0.0001)
P<0.05 in univariate models
Multiple Regression Analysis for Independent Variables
Dependent variables were entered into a multiple regression selection
procedure to identify independently significant predictors of IC50 values. In this
case, the final model contained the following terms:
_--Tropism (p<0.0001)
_--ART Naive (p=0.0104)
_--CD4+ Cell Count (p=0.0065)
_--Plasma HIV-1 RNA fell out of significance in this analysis
|
| |
|
 |
 |
|
|