 |
 |
 |
| |
Nevirapine Resistance in Mother-To-Child Transmission
|
| |
Reported by Jules Levin
In today's opening morning session at the workshop titled "Mother To child Transmission", there were 4 oral presentations of studies looking at nevirapine and reducing HIV transmission & reducing nevirapine resistance to mother & infant.
"Infant NVP Resistance Can be Substantially Reduced After Single Dose NVP by Avoiding Maternal NVP Dosing and Providing Infants with AZT in Addition to Single Dose NVP After Birth"
The objective of this study is to compare the rates of NVP resistance among infants in the NVAZ study who were exposed to different maternal/infant prophylactic regimens than HIVNET 012, single-dose NVP for early presenting mother & infant. Eshleman concluded: avoiding maternal NVP and providing infants with NVP+AZT was optimal of the 4 regimens. NVP resistance in women was eliminated. NVP resistance in infants was dramatically reduced: 87% (HIVNET 012) vs 27% group 4 regimen), p<0.001, by avoiding pre-delivery maternal NVP and providing infants with nevirapine+AZT (14-15%). The risk of HIV-1 MTCT was comparable to HIVNET 012 and another regimen that administered NVP to the mothers. These results are encouraging, but require confirmation in other studies.
Susan Eshleman (Johns Hopkins) provided this background. Administration of single dose NVP to women in labor and infants after birth can prevent HIV-1 MTCT. However, NVP-based regimens for prevention of MTCT are associated with emergence of NVP resistance in some women and some infants who are HIV-infected despite prophylaxis. This may compromise use of the regimen in subsequent pregnancies and/or treatment of women and infants with NNRTI-based regimens.
SD (single dose) NVP AND DRUG RESISTANCE
"The more you look, the more you find"
Johnson et al: The rate of NVP resistance in women after SD NVP is underestimated by routine genotyping
Flys et al: NVP-resistant variants persist in some women and infants above baseline levels for a year or more after SD NVP
Eshleman et al: The rate of NVP resistance is even higher in women with subtype C than in other subtypes
JID In Press 7/05
Other approaches for prevention of HIV MTCT are needed to: (1) reduce NVP resistance in women & infants; (2) provide prophylaxis for women who present late in labor or after delivery; (3) reduce HIV-1 transmission post-partum (breast-feeding).
This study analyzed NVP resistance 6-8 weeks after single-dose NVP in 78 infants who became HIV-infected despite antiretroviral prophylaxis.
The NVAZ trial in Malawi compared 4 regimens for prevention of HIV-1 mother-to-child transmission:
--group 1: women and infants received single-dose NVP (HIVNET 012 regimen);
--group 2: women received single-dose NVP, infants received single-dose NVP+AZT twice a day for 7 days
--group 3: women (presenting in late labor) received no NVP, infants received single-dose NVP;
group 4: women presenting in late labor received no NVP, infants received single-dose NVP+AZT as above.
The HIV-1 transmission rate was similar for groups 1, 2, and 4:
Group 1: 14.1% overall; incident 6.5%
Group 2: 16.3% overall; 6.9% incident
Group 3: 20.9% overall; incident 12.1%
Group 4: 15.3% overall; 7.7% incident
Lancet 2003;362:1171-7
JAMA 2004;292:202-9
High Rates of Resistance were observed in women & infants who received the HIVNET 012 regimen (subtype C):
GROUP 1: NVP RESISTANCE at Weeks 6-8: 69% for women; 87% for infants.
RESULTS
Group 1: 87% (20/23) of infants with NVP resistance; OR 22.24
(Women SD NVP; Infants (SD NVP)
Group 2: 74% (14/19) of infants with NVP resistance; OR 8.38
(Women SD NVP; Infants SD NVP+AZT)
Group 3: 57% of infants (12/21) with NVP resistance; OR 5.07
(Women no NVP; Infant SD NVP)
Group 4: 27% (4/15) of infants with NVP resistance; OR 1.00
(women no NVP; Infants SD NVP+AZT)
OR adjusted for maternal viral load.
"Single Dose Nevirapine Combined with a Short Course of Combivir for Prevention of Mother To Child Transmission of HIV-1 Can Significantly Decrease the Subsequent Development of Maternal and Infant Resistant Virus"
David Hall (Boehringer Ingelheim) reported results from a trial of singl-dose NVP+short course AZT.
Study participants were randomly assigned to 1 of 3 regimens:
--mother: SD NVP 200mg during labor
Infant: SD NVP 2 mg/kg
--mother SD NVP + 4 days Combivir
Infant: SD NVP + 4 days AZT+3TC
--mother: SD NVP + 7 days Combivir
Infant: SD NVP + 7 days AZT+3TC
NNRTI Mutations: K101E, K103N, V106AM, Y181C, Y188C, G190A.
MATERNAL RESISTANCE
Total with mutations:
NVP (68 treated): 41 (60%)
NVP/CBV4 days (67 treated): 8 (12%)
NVP/CBV7 days (68 treated): 7 (10%)
MEDIAN HIV RNA AT BASELINE & AT NADIR FOR MOTHERS:
NVP: 23,000 baseline; 8300 nadir viral load
NVP/CBV 4 days: 24,000; <400 nadir viral load
NVP/CBV 7 days: 35,000; 438 nadir viral load
INFANT RESISTANCE
Total with NNRTI mutations:
NVP (8 infected in utero): 7 (88%)
NVP/CBV 4 days (7 infected in utero): 1 (14%)
NVP/CBV 7 days (7 infected in utero): 0 (0%)
OBSERVATIONS:
Single dose NVP & 4 or 7 days of Combivir can produce a transient reduction of plasma HIV RNA to <400 copies/ml & avoid 80% of the emergence of NNRTI mutations seen with single dose NVP. There was no evidence of emergence of 3TC associated mutations at codon 184.
With SD NVP, NNRTI associated mutations emerge at different rates & remain for different durations. The pattern of mutations observed will depend on when samples are collected. Mutations were observed for the first time as late as 25 weeks.
CONCLUSIONS:
A short course of Combivir significantly reduces the emergence of NVP-associated mutations in mothers taking single-dose NVP to prevent mother-to-child transmission of HIV-1.
A short course of Combivir reduces the emergence of NVP-associated mutations in infants taking SD single dose NVP after intrauterine infection.
"Short Course Combivir After Single Dose Nevirapine Reduces But Does Not eliminate the Selection of Nevirapine-Resistant HIV"
Despite the positive findings reported above Sarah Palmer reported drug resistance was more prevalent using a sensitive resistance assay. The objective of this study was to use a sensitive allele-specific RT-PCR assay to determine the frequency of NNRTI-resistant mutants in women who had received single-dose nevirapine alone or in combination with Combivir for 4 or 7 days.
This assay detects & quantifies all alleles, variants encoding 181C or 103N, down to 0.1%.
32 women enrolled in BI study:
--10 received single-dose NVP
--11 received single-dose NVP + 4 days CBV
--11 received single-dose NVP + 7 days CBV
Plasma samples were collected at:
Baseline (pre-treatment), 2 weeks, 6 weeks
PERCENT of WOMEN with NNRTI MUTATIONS at WEEK 6
Using the sensitive test the percent of women with NNRTI mutations was higher than found when using the standard genotype test: 50% vs 75% (NVP); 0% vs 27% (sdNVP+CBV-4 days); 0% vs 27% (sdNVP+CBV-7 days).
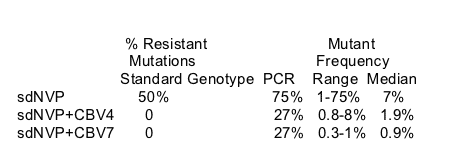
Palmer concluded:
Six weeks after sdNVP alone, 75% of women had NNRTI-resistant variants in plasma detectable by allele-specific PCR.
Short course CNV for 4 or 7 days reduced this frequency to 27% of women at week 6.
At week 6, the median fraction of plasma virus that comprises 103N (AAC) & 181c variants appears to be lower in women who received sdNVP + CBV (4 or 7 days) versus sdNVP alone.
The frequency of NNRTI resistant variants selected by sdNVP with or without CBV varies among women.
NNRTi resistant variants, even at low frequencies, may compromise future treatments containing NNRTIs and limit the subsequent efficacy of sdNVP fpr pMTCT.
Additional studies are needed to:
--establish the clinical significance of low frequency NNRTI resistant vatiants
--define the optimal duration of CBV to eliminate the selection of NNRTI resistant variants
"Patterns of viral load and drug resistance in breastmilk and blood from women treated with single-dose nevirapine to reduce mother-to-child transmission of HIV-1"
DA Lehman1,3, MH Chung4, BA Richardson2,
GC John-Stewart4,5 and J Overbaugh1,3
1Division of Human Biology, Fred Hutchinson Cancer Research
Center, Seattle, USA
2Division of Public Health Sciences, Fred Hutchinson Cancer
Research Center, Seattle, USA
3Department of Molecular and Cellular Biology, University of
Washington, Seattle, USA
4Department of Medicine. University of Washington, Seattle,
USA
5Department of Epidemiology, University of Washington, Seattle,
USA
BACKGROUND: The use of single-dose nevirapine (NVP) to reduce mother-to-child transmission of HIV-1 is increasing in the developing world. However, little
is known regarding the effects of this regimen on viral loads in breast milk or on the temporal patterns of drug resistance that arise during the early postpartum
period. These are important to determine because considerable HIV-1 transmission occurs during the first 6 weeks postpartum in breastfeeding populations. We have determined the effect of NVP on breast milk viral
shedding and are examining the prevalence of NVP resistance during this early postpartum period.
METHODS: Thirty pregnant HIV-1 seropositive women in Nairobi, Kenya who planned to breastfeed were treated with the single-dose NVP regimen
(HIVNET 012). Two to four breast milk samples were collected each week between delivery and 6 weeks postpartum. Breast milk HIV-1 RNA was quantified using the Gen-Probe HIV-1 viral load assay. HIV-1 DNA was extracted from blood samples collected at 1 month postpartum and was tested for NVP resistance using an allele-specific PCR assay that detects the K103N mutation.
RESULTS: Breast milk viral loads in 30 women treated with single-dose NVP were compared to breast milk viral loads from 30 women treated with the Thai-CDC short-course zidovudine regimen. A total of 404 breast milk samples from the NVP-treated women were tested, with a median of 14 samples per woman during the first 6 weeks postpartum. Between 3 and 21 days postpartum,
treatment with NVP was associated with significantly greater suppression of breast milk log10 HIV-1 RNA: days 3 to 7 (1.98 vs 2.42, P=0.1); days 8
to 14 (1.78 vs 2.48, P=0.005); days 15 to 21 (1.90 vs 2.97, P=0.003). In preliminary studies, utilizing an allele-specific PCR assay, we tested one-month postpartum blood samples from the NVP-treated women and determined that 40% of the women tested to date had detectable levels of the K103N mutation. Breast milk samples are currently being tested.
CONCLUSIONS: Compared to the Thai-CDC shortcourse zidovudine regimen, single-dose NVP results in sustained suppression of breast milk viral loads during the first 3 weeks postpartum. However, the benefits of this suppression may be counterbalanced with a high prevalence of resistance.
| |
| |
| |
| |
|
 |
 |
|
|