 |
 |
 |
| |
Tipranavir: predicting viral response
|
| |
Poster presented at 14th HIV Drug Resistance Workshop, June 7-11, 2005, Quebec City, Canada
"Non-response to Tipranavir is Associated with Pretreatment Resistance Characterized by Tipranavir Phenotype or Genotypic Tipranavir Score"
--this study analysis examines the relationship between non-response and baseline resistance to TPV was explored. Reduced susceptibility or high TPV score were associated with a greater non-response rate; however, the majority of patients still responded to TPV (86%). The majority of patients who include a new drug in their TPV-containing regimen are able to maintain an antiviral response of >1 log10 for 24 weeks. T-20 improves viral response & durability.
Authors: H Valdez1, DB Hall1, VM Kohlbrenner1, CA Boucher2, J Schapiro3, J Baxter4, J Scherer1, S McCallister1, DL Mayers1
1Boehringer Ingelheim Pharmaceuticals, Inc. Ridgefield, CT, USA; 2University of Utrecht, Utrecht, The Netherlands; 3Stanford University, Stanford, CA, USA; 2Cooper Hospital, Camden, NJ, USA.
INTRODUCTION
Tipranavir (TPV) is a non-peptidic protease inhibitor (NPPI) with a resistance profile distinct from currently available PIs. It retains broad and potent in vitro antiviral activity against protease-resistant HIV isolates. In vivo TPV/r treatment of HIV naive and experienced patients is well tolerated and achieves a potent and durable antiviral activity.1-4
The Phase III trials, RESIST-1 and RESIST-2, are international, randomized, open-label studies in HIV-positive 2-PI-experienced patients designed to compare the efficacy and safety of TPV/r and comparator PI (CPI). Analyses of these 2 trials at 24 weeks have demonstrated that TPV/r was superior to CPI/r.5-6 The analyses presented here evaluate the small proportion of patients on TPV/r in the RESIST trials who had no response to treatment, defined as less than a 0.5 log10 drop from baseline in HIV-1 RNA during the first 8 weeks of treatment. Additionally, to evaluate the full spectrum of impact of resistance on treatment outcome, we have expanded the analyses to include all data from the TPV development program from patients treated with the to-be-marketed TPV 500 mg boosted with RTV 200 mg BID dose.
ABSTRACT
BACKGROUND
In Phase III RESIST trials, tipranavir (TPV) with ritonavir boosting was introduced along with an optimized background regimen that could include enfuvirtide and any of the licensed antiretroviral nucleoside and non-nucleoside reverse transcriptase inhibitors. A small proportion of patients on TPV (13.8%) had no response to their study regimen with no more than a 0.5 log10 drop from
baseline in HIV-1 RNA during the first 8 weeks of treatment. The relationship between non-response and baseline resistance to TPV was explored.
METHODS
Genotypic sequencing was performed by Virtual Phenotype or TRUGENE assays. Phenotypic assays were performed using the VIRCO Antivirogram and were available for 361 TPV/r-treated patients. To be eligible for the clinical trial patients had to have used at least two protease inhibitor-based HAART regimens and had to have at least one protease mutation from among 30N, 46I/L,
48V, 50V, 82A/F/L/T, 84V, 90M with not more than two mutations at codons 33, 82, 84, and 90.
Mutations were summarized in terms of the TPV score, consisting of the number of codons with mutations from among 10V, 13V, 20M/R, 33F, 35G, 36I, 43T, 46L, 47V, 54A/M/V, 58E, 69K, 74P, 82L/T, 83D, and 84V. The TPV score was derived using univariate and multivariate regression analyses of Phase II TPV clinical trial genotype to phenotype and genotype to virologic response data.
RESULTS
Phenotypic susceptibility to TPV was associated with virologic response, with 92% (233/252) response in those with baseline IC50-fold change <3 compared to 68% (74/109) in those with baseline IC50-fold change of 3 or greater.
Similarly the TPV score was strongly associated with response. Patients with baseline TPV scores of 2 or less had 94% response (225/239); with scores of 3 to 5 had 84% response (374/446); and with scores of 6 or more had 72% response (43/60).
CONCLUSIONS
IC50-fold change of <3 or a low TPV score predicted a response to TPV. Reduced susceptibility or high TPV score were associated with a greater non-response rate; however, the majority of patients still responded to TPV.
AUTHOR CONCLUSIONS
--TPV/r is virologically active against the majority of HIV-1 isolates with broad PI resistance
--The majority of patients (86.2%) of patients achieved a 0.5 log10 viral load reduction within 8 weeks of TPV/r treatment
--Patients with viruses with a >10-fold decrease in TPV susceptibility have decreased antiviral responses to TPV-containing regimens, but at least 2/3 of these patients achieve a 0.5 log10 viral load reduction within 8 weeks
--Patients with viruses with a TPV mutation score of ≥8 have decreased antiviral responses to TPV-containing regimens, but at least 3/4 of these patients achieve a 0.5 log10 viral load reduction within 8 weeks
--The TPV mutation score relates well with changes in baseline TPV susceptibility
--A relationship between TPV mutations score and change in TPV susceptibility has been established:

The interpretation of durability of virologic response data among patients whose HIV isolates have a high TPV mutation score is confounded by the decreasing
number of background drugs to support the antiviral activity of TPV
Patients in the TPV development program had multiple protease resistant isolates. The majority of these isolates are fully susceptible to TPV with IC50 <3
Very few patients had HIV isolates with TPV mutation scores of ≥8 or TPV change in IC50 of >10, however, most of these patients experienced a meaningful decrease in viral load after TPV administration
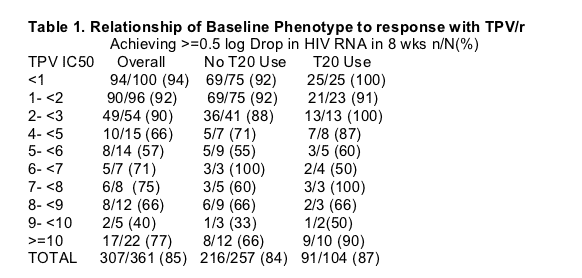
BASELINE PHENOTYPIC SUSCEPTIBILITY TO TPV IS
ASSOCIATED WITH VIROLOGIC RESPONSE IN RESIST TRIALS
Virologic response (≥0.5 log10 drop in viral load within 8 weeks) was strongly and significantly (P<0.001, Chi-squared test) related with TPV IC50 of <3 as
compared to ≥3 (Table 1):
--92% of patients (233/252 patients) whose isolates had a baseline TPV IC50-fold change <3 achieved virologic response
--68% of patients (74/109) whose isolates had a baseline TPV IC50-fold change ≥3 achieved virologic response
Virologic response among patients with TPV IC50-fold change <3 was not dependent on T-20 use (Table 1):
--Virologic response was achieved by 96.7% (59/61) of T-20 recipients and 91.1% (174/191) non-T-20 recipients
BASELINE TPV SCORE IS ASSOCIATED WITH VIROLOGIC
RESPONSE IN RESIST TRIALS
Virologic response (≥0.5 log10 drop in viral load within 8 weeks) was strongly and significantly (P<0.001, Chi-squared for trend) related with TPV score:
--94% (225/239) of patients whose baseline isolate had TPV scores of =2 achieved virologic response
--84% (374/446) of patients whose baseline isolate had TPV scores of 3-5 achieved virologic response
--72% (43/60) of patients whose baseline isolate had TPV scores of ≥6 achieved virologic response
Virologic response among patients with TPV score =2 was not dependent on T-20 use:
--Virologic response was achieved by 94.9% (37/39) of T-20 recipients and 94.0% (188/200) non-T-20 recipients
RELATIONSHIP BETWEEN TPV SCORE, TPV PHENOTYPIC
SUSCEPTIBILITY, AND ANTIVIRAL RESPONSE
For the following sections, all data available for patients who received TPV/r at a dose of 500 mg/200 mg in the TPV development program are used to expand the
breadth of baseline TPV susceptibility.
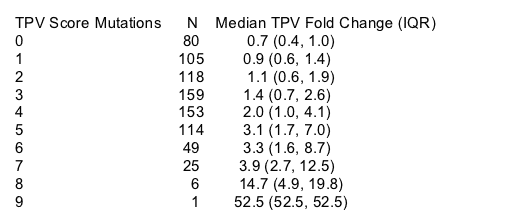
Relationship of TPV score to TPV phenotype (Table 3)
--Patients whose baseline isolates have a TPV score between 0 and 4 have median TPV IC50 of <3
_--Patients whose baseline isolates have a TPV score between 5 and 7 have median TPV IC50 of >3 but <10
--Patients whose baseline isolates have a TPV score of 8 or more have median TPV IC50 of >10
Table 3. Median TPV IC50-Fold Change from Wild-Type by Number of TPV Score Mutations among Participants in the TPV Development Program
Relationship of TPV score to antiviral activity of a TPV-containing regimen (Table 4)
--Median viral load change at 2 weeks in patients with TPV score <8 is > 1 log10
--Median week 24 viral load change in patients with a TPV score <2 is > 2 log10
--Median week 24 viral load change in patients with a TPV score <8 is between 0.5 and 1 log10

T20 IMROVES VIRAL RESPONSE & DURABILITY
DURABILITY OF ANTIVIRAL RESPONSE ACHIEVED
WITH TPV
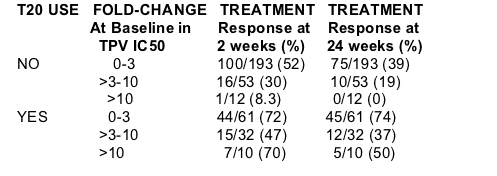
Durability of antiviral response by TPV susceptibility and T-20 use
The majority of patients who include a new drug in their TPV-containing regimen are able to maintain an antiviral response of >1 log10 for 24 weeks (Table 5)
--All patients whose baseline isolates had a TPV IC50 ≦3 and achieved a virologic response at week 2 maintained their virologic responses through 24
weeks when TPV/r and T-20 were used
--80% (12/15) of patients whose baseline isolates had a TPV IC50 > 3 but ≦10 and achieved a virologic response at week 2 maintained their virologic
responses through 24 weeks when TPV/r and T-20 were used
--71% (5/7) of patients whose baseline isolates had a TPV IC50 >10 and achieved a virologic response at week 2 maintained their virologic responses
through 24 weeks when TPV/r and T-20 were used
Table 5. Percentage of TPV/r recipients in RESIST who achieve a 1 log10 viral load reduction at weeks 2 or 24 according to baseline TPV susceptibility and T20
use
TPV score and background ARVs
The majority of patients with a TPV score <3 was able to include ≥2 reverse transcriptase inhibitors (RTI) in their background regimen while the majority of
those with a baseline TPV score ≥3 included 1 or fewer active RTIs in their regimen.
Decreased durability of antiviral responses to a TPV-containing regimen in patients with a high TPV score is due to lack of an effective background regimen.
REFERENCES
1. Back NK, van Wijk A, Remmerswaal D, et al. AIDS. 2000;14:101-102.
2. Larder BA, Hertogs K, Bloor S, et al. AIDS. 2000;14:1943-1948.
3. McCallister S, Valdez H, Curry K, et al. J Acquir Immune Defic Syndr. 2004;35:376-382.
4. Gathe J, Kohlbrenner VM, Pierone G, et al. Presented at: 10th CROI; February 10-14, 2003; Boston, Mass.
5. Hicks C, for the RESIST-1 Study Team. Presented at: 44th ICAAC; October 30-November 2, 2004; Washington, DC.
6. Cahn P, and the RESIST-2 Study Team. Presented at: 7th International Congress on Drug Therapy in HIV infection; November 14-18, 2004; Glasgow, Scotland.
7. Kohlbrenner VM, Hall DB, Baxter J, et al. Tipranavir (TPV) Mutation Score Predicts Virologic Success in PI-Experienced HIV-Positive Adults. Presented at: HIV DART 2004; December 12-16, 2004; Montego Bay, Jamaica.
| |
| |
| |
| |
|
 |
 |
|
|