 |
 |
 |
| |
Two-Year Results from the GLOBE Trial in Patients with Hepatitis B: Greater Clinical and Antiviral Efficacy for Telbivudine (LdT) vs Lamivudine
|
| |
| |
C-L Lai, E Gane, C-W Hsu, S Thongsawat, Y Wang, Y Chen , EJ Heathcote, J Rasenack , N Bzowej, N Naoumov, S Zeuzem, A Di Bisceglie, GC Chao,
B Fielman Constance, NA Brown and the Globe Study Group
AASLD Annual Meeting
October 27 - 31, 2006
Boston, Massachusetts
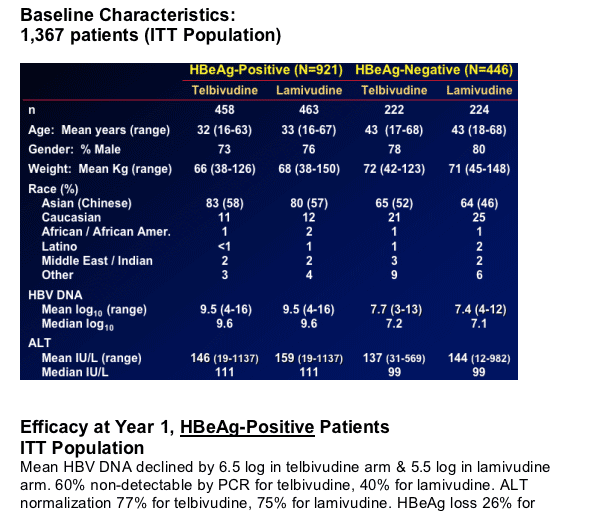
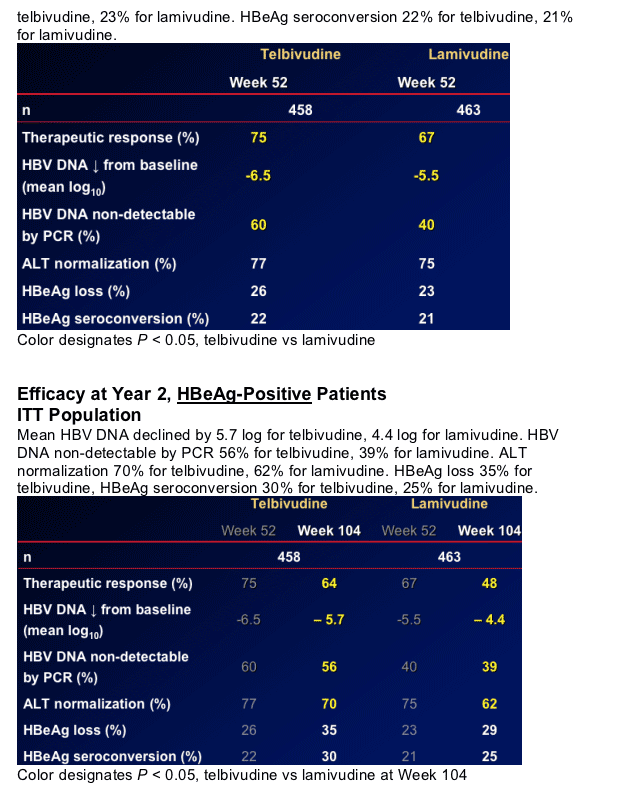
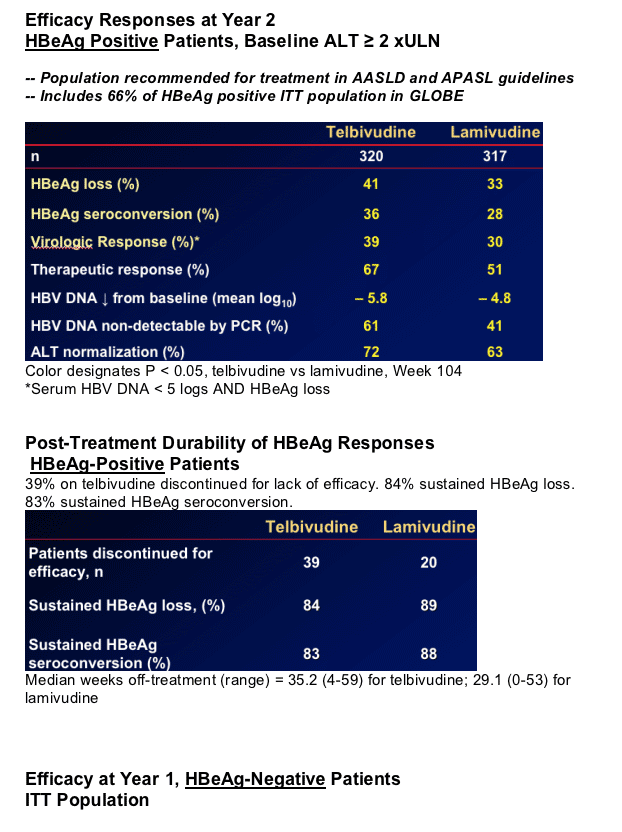
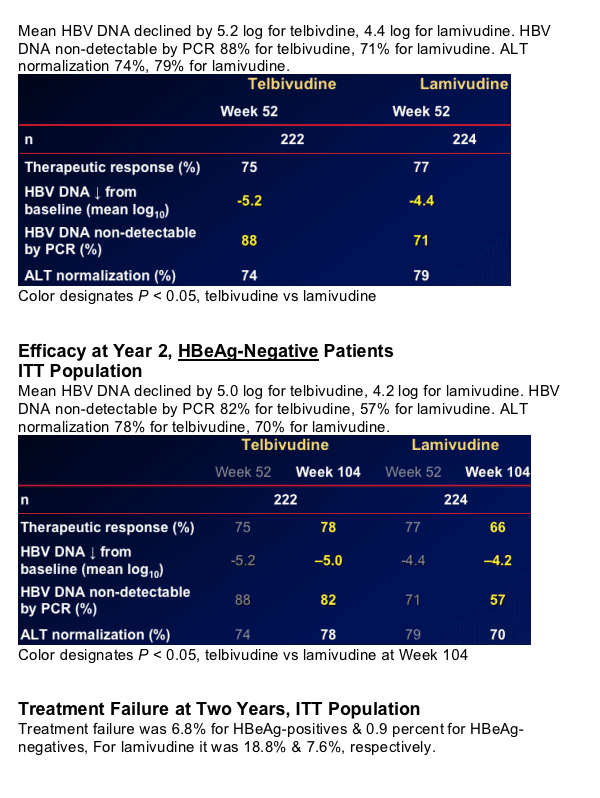
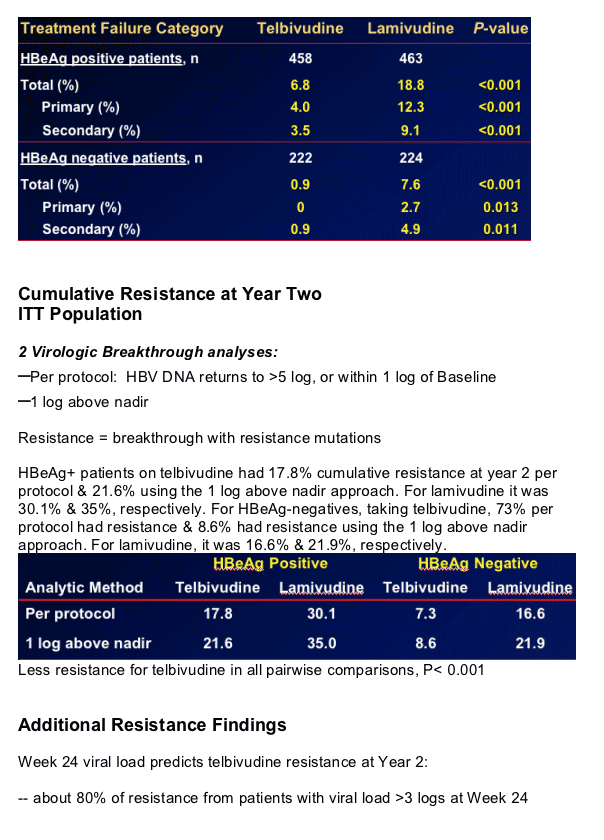
Brief Summary: after 2 years mean HBV DNA decline in HBeAg-positives was 5.7 log for telbivudine & 4.4 log for lamivudine. ALT normalization was 70% for patients taking telbivudine & 62% for lamivudine. HBeAg loss was 35% for patients taking telbivudine & 29% for lamivudine. HBeAg-seroconversion was 30% for patients taking telbivudine ^ 25% for patients taking lamivudine. For HBeAg-negatives mean HBV DNA decline was 5.0 log for telbivudine & 4.2 for lamivudine. HBV DNA non-detectable was 82% for telbivudine & 57% for lamivudine. Two-year virologic breakthrough (resistance) was 17.8-21.6% for HBeAg-positives taking telbivudine and 7.3-8.6% for HBeAg-negatives.
Telbivudine (LdT)
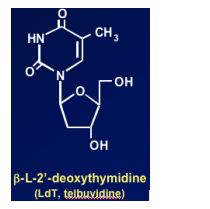
Specific inhibitor of HBV polymerase
-- Not active against HIV or other viruses
Favorable toxicology for long-term treatment:
-- Non-mutagenic, non-carcinogenic, no mitochondrial toxicity
-- No preclinical teratogenicity or embryofetal toxicities
Once daily oral dosing indicated by PK
-- Consistent absorption, no food effect
First year of Phase III GLOBE comparative trial1
-- Greater antiviral effect vs lamivudine in eAg+ and eAg- patients, with less resistance and good safety
1 Lai et al., AASLD 2005 (Hepatology 2005;42(4 Suppl. 1):748A)
GLOBE Study Design
Entry criteria:
-- Chronic hepatitis B with compensated liver disease, HBeAg+ or HBeAg-
-- Serum HBV DNA > 106 copies/mL by COBAS Amplicor PCR assay
-- ALT ≥ 1.3 xULN and < 10 xULN
-- Pre-treatment liver biopsy consistent with chronic hepatitis B
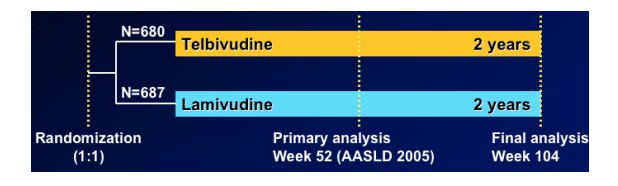
Enrolled ITT population: 1,367 patients
-- 921 HBeAg-positive, and 446 HBeAg-negative
GLOBE Analyses at Year Two (Week 104)
Methods:
ITT analyses through Week 104, outcomes for all 1367 patients
Missing = failure for categorical endpoints
Key categorical responses analyzed as maintained responses
-- ≥ 2 consecutive qualifying values including last visit
-- Pre-specified analysis of predictors of response (DiBisceglie, 3:45PM Monday)
GLOBE Key Efficacy Endpoints
Therapeutic Response (primary efficacy endpoint)
-- HBV DNA ≦105 copies/mL, AND ALT normalized or HBeAg loss
Antiviral efficacy
-- HBV DNA reduction from baseline
-- Clearance of HBV DNA to PCR-nondetectable
ALT normalization
HBeAg responses (HBeAg+ patients only)
Liver histology not evaluated at two years
Safety Summary
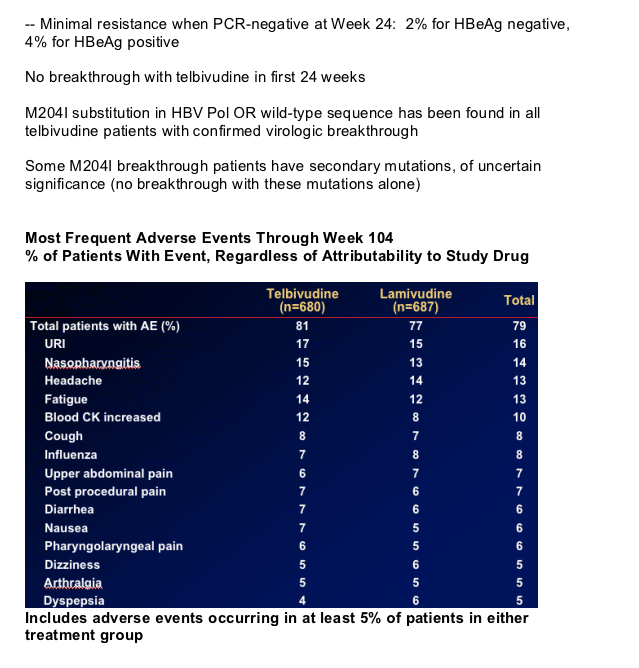
Clinical Adverse Events:
-- Overall similar pattern of adverse events for both treatments
ALT elevations
More common with lamivudine vs telbivudine, esp. after Wk 24
Moderate-severe ALT flares (ALT> 500 IU/mL, or flare with bilirubin 2 xULN):
-- 2.8% for LdT vs 8.4% for Lam (overall population, W24-104)
-- 1 Lam recipient required liver transplant
Creatine Kinase elevations
- CK elevations: typically asymptomatic or non-specific symptoms
3 cases of mild-moderate myopathy/myositis on telbivudine
(all resolved) suggest persistent, unexplained muscle-related symptoms should be evaluated promptly
Conclusions:
Telbivudine vs. lamivudine in HBeAg positive and negative patients at 2 Years
Telbivudine showed significantly greater:
-- % Therapeutic Response
-- HBV DNA log10 reduction from baseline
-- % HBV DNA PCR negative and more rapid time to PCR-negative
-- HBeAg response rate in patients with population with baseline ALT ≥ 2 xULN
Telbivudine showed significantly less:
-- Primary and secondary treatment failure
-- Breakthrough and resistance
Similar overall clinical adverse event profiles, telbivudine and lamivudine
Post-treatment durability of HBeAg loss or seroconversion >80%
Low resistance at 2 years in patients with optimal early viral suppression
Favorable preclinical toxicology
|
| |
|
 |
 |
|
|