 |
 |
 |
| |
The cyclophilin inhibitor DEBIO-025 has a potent dual anti-HIV and anti-HCV activity in treatment-naive HIV/HCV co-infected subjects
|
| |
| |
Reported by Jules Levin
AASLD, Oct 27-31, 2006, Boston, MA
R Flisiak1, A Horban2, J Kierkus3, J Stanczak2, I Cielniak2, GP Stanczak2, A Wiercinska-Drapalo1, E Siwak1, J Higersberger2, C Aeschlimann4,
P Grosgurin4, V Nicolas4, J-M Dumont4, H Porchet4, R Crabbe4, P Scalfaro4
1 Department of Infectious Diseases, Medical University of Bialystok, Bialystok, Poland; 2Hospital of Infectious Diseases, Warsaw, Poland;
3Department of Gastroenterology, Hepatology and Immunology, The Children's Memorial Health Institute, Warsaw, Poland; 4Debiopharm SA, Lausanne, Switzerland
DEBIO-025, a cyclophilin inhibitor, demonstrated strong antiviral activity in vitro against HCV1 and HIV-1. In a previous phase I study, DEBIO-025 showed antiviral effect (< 1 Log10 reduction) in HIV-1-positive asymptomatic subjects treated with DEBIO-025 400 and 1200 mg daily for 10 days2.
This phase I study aimed to determine the antiviral effect, pharmacokinetic (PK) profile and safety of DEBIO-025 in treatment-naive HIV/HCV co-infected subjects.
I spoke with the study investigator at AASLD, and due to the safety issues a lower dose wil be examined in the next study.
SAFETY
Total bilirubin increased by a median of 22 _mol/L (range -1 to +88) during treatment, leading to hyperbilirubinaemia in 10 subjects. A total of 4/19 (21%) subjects discontinued DEBIO-025 treatment prematurely for this reason. No increase in ALT/AST or _-GT was observed, and there were no signs of haemolysis. Bilirubin returned rapidly to baseline levels after treatment cessation. This phenomenon was not observed in previous DEBIO-025 studies at doses up to 1200 mg OD for 10 days. A saturation of biliary canalicular transporters by high concentrations of DEBIO-025 causing a competitive inhibition of bilirubin excretion is a probable explanation for this phenomenon. A median change in
thrombocytes of -49 109/L (range -178 to - 4) was observed in the DEBIO-025 group v.s. -17 109/L (range -38 to +26) in the placebo group. "Decreased platelets" was reported as an adverse event in 2/19 subjects on DEBIO-025, but was not associated with signs of increased bleeding and subjects continued study treatment. After treatment completion, platelets returned rapidly to baseline levels.
AUTHOR CONCLUSIONS
DEBIO-025 monotherapy for 15 days induced an important anti-HCV antiviral effect (3.6 Log10 reduction) in HIV-HCV co-infected subjects, a population generally responding poorly to anti-HCV treatment. The antiviral effect was consistent throughout treatment (no breakthrough) on the 3 genotypes identified in the study. DEBIO-025 also showed some direct anti-HIV-1 effect (1 Log10 reduction) through a novel mode of action that may differentiate it from other anti-HCV compounds in this patient population. Reversible and dose-dependent hyperbilirubinaemia, probably caused by saturation of biliary canalicular transporters, seems to be the dose limiting factor for DEBIO-025.
METHODS
This was a double-blind, placebo-controlled, oral dosing, phase Ib study. Treatment-naive HIV-1 mono or HIV/HCV co-infected subjects received 1200 mg DEBIO-025 or placebo twice daily for 15 days (unbalanced randomization).
DEBIO-025 plasma PK was assessed by LC/MS on Days 1 and 15. Viral
loads were determined with the Abbott Realtime m2000 PCR system (HCV limit of quantification [LOQ] = 43 copies/mL; HIV-1 LOQ = 25 copies/mL) on Day -1, 12 hours after the first dosing, before the morning dose on Days 2, 4, 8, 10 and 15, and at week 1, 2 and 3 after the last dose.
RESULTS
Population
Twenty-three Caucasian subjects completed the study. Nineteen subjects aged 19-55 years received DEBIO-025, whereas 4 subjects received placebo (age range 26-33 years). The overall study population was predominantly male (18/23; 78.3%). A total of 19/23 of subjects (82.6%) were co-infected with HIV-1 and HCV, whilst the remaining subjects were mono-infected with HIV-1. HCV genotypes in the 16 HIV-1/HCV co-infected subjects on active treatment were evenly distributed between genotype 1 (n=5), genotype 3 (n=6), and genotype 4 (n=5).
Pharmacodynamics
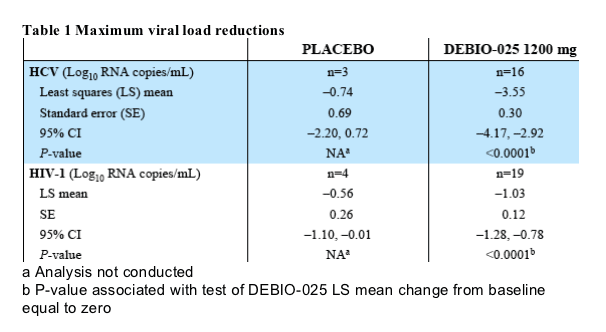
HIV-1/HCV co-infected DEBIO-025-treated subjects experienced a significantly greater maximum HCV viral load reduction compared to placebo subjects, achieving a least squares mean of 3.6 vs. 0.7, respectively [P=0.0018 (ANCOVA with pre-treatment viral load as covariate)] (Table 1). The difference between treatment groups was also significant when calculated from Day -1 to Day 10 (P= 0.0046) and from Day -1 to Day 15 (P= 0.0066) (data not shown), suggesting a consistent effect over the entire treatment duration. A total of 15/16 subjects (93.8%) in the DEBIO-025 group achieved a HCV viral load reduction of at least 2 Log10. HCV RNA became undetectable in 3 subjects during the treatment period.
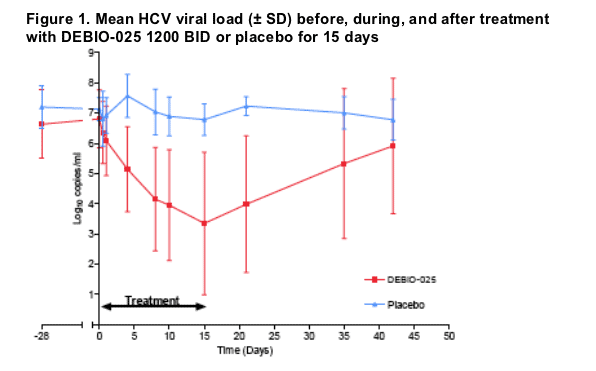
Figure 1 shows that subjects on placebo did not change their HCV viral load during treatment. The overall subject population on active treatment showed an important and continuous viral load decline during DEBIO-025 treatment without reaching a plateau. After treatment cessation, HCV viral load levels returned gradually to baseline values over the 4-week follow up period.
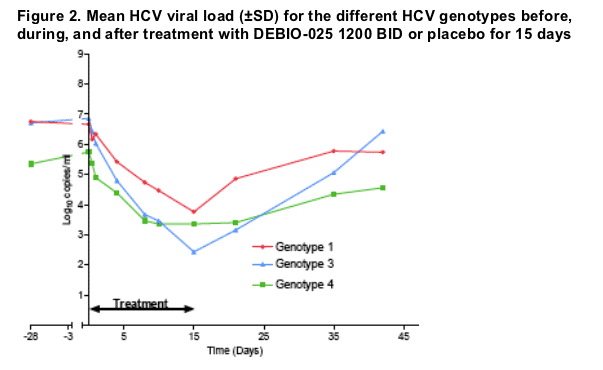
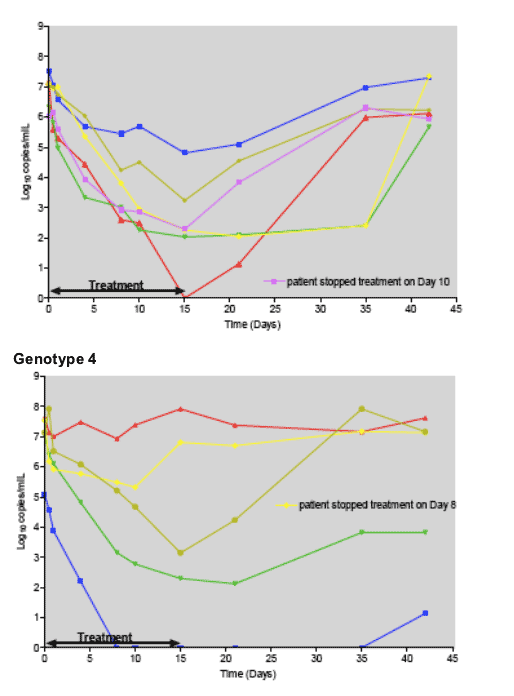
The 3 HCV genotypes identified in the study responded well to the dose administered (Figure 2). Because of the small number of subjects in each genotype subgroup it is not possible to draw conclusions about a possible difference in sensitivity to treatment.
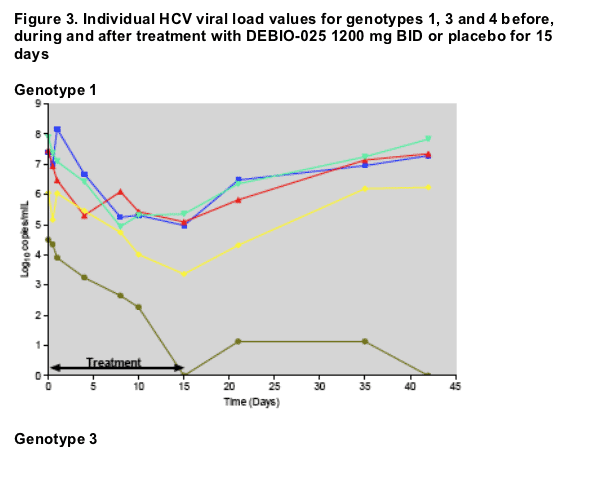
Individual subject data show that all but one null-responder (genotype 4) achieved at least a 2 Log10 drop in HCV viral load during treatment. Three subjects (one of each genotype) decreased viral loads below detectable levels at treatment Day 15 (2 subjects) and Day 8 (1 subject). No single subject developed a breakthrough phenomenon during treatment. All subjects who experienced a relapse increased their HCV viral load levels after treatment cessation only. A time to relapse as long as 3 weeks was observed in 3 subjects, although time to relapse was overall highly variable.
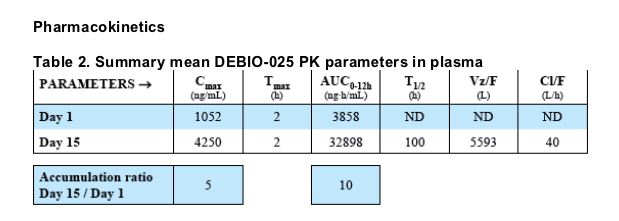
DEBIO-025 was rapidly absorbed, with peak plasma levels reached after ca. 2 hours, and exhibited a multiphasic elimination with a mean terminal half-life of 100 hours. An important accumulation of the drug over time was observed with Cmax and AUC increasing 5 and 10 fold, respectively, after 2 weeks of treatment.
References
1 Paeshuyse J et al. The Non-Immunosuppressive Cyclosporin DEBIO-025 Is a Potent Inhibitor of Hepatitis C Virus Replication In vitro. Hepatology 2006;43:761-70
2 Steyn D et al. A Double-Blind Placebo-Controlled Study in HIV-1- infected Subjects on the Safety, Pharmacokinetics and Antiviral Effect of Cyclophilin A Targeting DEBIO-025 [poster]. CROI; 2006 Feb 5-8; Denver, USA.
R Flisiak, A Horban, J Kierkus, J Stanczak, I Cielnak, GP Stanczak, A Wiercinska-Drapalo, E Siwak, and J Higersberger have received financial support from Debiopharm S.A. for their participation in the study, but have no other financial interests in this work.
C Aeschlimann, P Grosgurin, V Nicolas, J-M Dumont, H Porchet, R Crabbe, and P Scalfaro are employed by Debiopharm S.A. Contact: Debiopharm SA, Lausanne, Switzerland: www.debiopharm.com.
Att. Raf Crabbe. E-mail: rcrabbe@debio.com. Fax: +41 (0)79 786 52 91
|
| |
|
 |
 |
|
|