 |
 |
 |
| |
Pegasys Every 5 Days in PegIntron Nonresponders
|
| |
| |
Reported by Jules Levin
AASLD, Oct 27-31, 2006, Boston, MA
"Efficacy and safety of peginterferon alfa-2a administered every 5 days in combination with ribavirin in HCV genotype 1-infected patients with severe fibrosis not responding to weekly administrations of pegylated interferon alfa-2b in combination with ribavirin"
C. Hezode,1,2 M. Bouvier-Alias,2,3 F. Roudot-Thoraval,2,4 E.-S. Zafrani,5 D. Dhumeaux,1,2 A. Mallat,1 J.-M. Pawlotsky2,3
1Department of Hepatology, Hopital Henri Mondor, Creteil, France; 2INSERM U635, Hopital Henri Mondor, Creteil, France; 3Department of Virology, Hopital Henri Mondor, Creteil, France; 4Department of Public Health, Hopital Henri Mondor, Creteil, France;
5Department of Pathology, Hopital Henri Mondor, Creteil, France
INTRODUCTION
The standard treatment for patients with chronic hepatitis C is the combination of a pegylated interferon plus ribavirin,[1] and overall sustained virologic response (SVR) rates of up to 66% have been achieved with peginterferon alfa-2a (40KD) (PEGASYS) plus ribavirin (COPEGUS) in large randomized international studies.[2-4] However, a substantial proportion of patients do not achieve an SVR with the standard regimen, particularly those with hepatitis C virus (HCV) genotype 1 and/or other 'difficult-to-cure' features such as cirrhosis/bridging fibrosis, and current treatment options in patients who have not achieved an SVR are limited.
A sustained response to treatment is dependent on maintaining viral suppression. Conversely, failure to respond to an initial course of therapy may be due to a rebound in viral replication. The results of a viral kinetic study have shown that serum concentrations of pegylated interferon alfa-2b (12KD) decline in the second half of the once-weekly dosing period, and this may result in serum drug levels that are too low to maintain viral suppression. In order to achieve constant serum levels of pegylated interferon alfa-2b (12KD), and to therefore improve the kinetics of viral suppression, it has been suggested that the drug should be administered on a twice-weekly basis.[5]
OBJECTIVE
The aim of this study was to evaluate the efficacy and safety of peginterferon
-2a (40KD) plus ribavirin in patients with severe fibrosis who failed treatment with weekly administrations of pegylated interferon alfa-2b (12KD) plus standard-dose ribavirin.
AUTHOR CONCLUSIONS
Peginterferon alfa-2a (40KD) (PEGASYS) plus ribavirin (COPEGUS) was effective and well tolerated in the retreatment of HCV genotype 1 patients with
severe fibrosis who had failed to respond to weekly pegylated interferon alfa-2b (12KD) plus ribavirin.
It remains unclear whether a fixed-dose induction of peginterferon alfa-2a (40KD)
administered on a once-weekly basis would not have produced the same antiviral effect observed in this study. However, such a regimen is currently being evaluated in the ongoing REPEAT study. REPEAT is investigating the use of a fixed-dose induction of peginterferon alfa-2a (40KD) for the first 12 weeks of
treatment and/or an extended duration of treatment (72 weeks) in non-responders (>90% genotype 1, 27% with cirrhosis/bridging fibrosis) to 312 weeks of pegylated interferon alfa-2b (12KD) plus ribavirin.[6]
METHODS
Patients
Patients included in this study had HCV genotype 1 infection, severe fibrosis (METAVIR grade F3) and had not cleared HCV following treatment with
once-weekly pegylated interferon alfa-2b (12KD) plus ribavirin.
- non-response to previous pegylated interferon alfa-2b (12KD) plus ribavirin therapy was defined as a) absence of a 32-log10 decline in HCV RNA between treatment initiation and 12 weeks (Versant HCV RNA 3.0 Assay [bDNA], lower limit of quantitation 615 IU/mL [2.79 log10 IU/mL]) and b) detectable serum HCV RNA after 24 weeks' treatment (COBAS AMPLICOR HCV Test, v2.0, lower limit of detection 50 IU/mL [1.70 log10 IU/mL]).
Study design
Patients were retreated with peginterferon alfa-2a (40KD) 180 ug administered every 5 days for 12 weeks, followed by 180 _g once weekly for an additional 36 weeks, in combination with weight-based ribavirin (1000 mg/day in patients weighing <75 kg and 1200 mg/day in patients weighing >75 kg) for 48 weeks.
- retreatment was initiated 6 months after pegylated interferon alfa-2b (12KD) plus ribavirin therapy was terminated.
Assessment of efficacy and safety
HCV RNA level was assessed by quantitative PCR assay (Versant HCV RNA 3.0 Assay [bDNA], lower limit of quantitation 615 IU/mL [2.79 log10 IU/mL]) after 4, 12, 24, 48 and 72 weeks' retreatment with peginterferon alfa-2a (40KD) plus ribavirin. In those patients with an HCV RNA level below the level of quantitation, HCV RNA was reassessed by qualitative PCR assay (COBAS AMPLICOR HCV Test, v2.0, lower limit of detection 50 IU/mL).
Decline in HCV RNA level from baseline following retreatment with peginterferon alfa-2a (40KD) plus ribavirin was compared with decline in HCV RNA from baseline after 12 and 24 weeks' treatment with peginterferon alfa-2b (12KD) plus ribavirin (initial treatment).
The rates of virologic response after 12 and 48 weeks' retreatment with peginterferon alfa-2a (40KD) plus ribavirin were determined.
- a response at week 12 was defined as a >2-log10 drop in HCV RNA, while a response at week 48 (end-of-treatment) was defined as undetectable (<50 IU/mL) HCV RNA.
SVR, defined as undetectable HCV RNA by PCR assay (COBAS AMPLICOR HCV Test, v2.0, lower limit of detection 50 IU/mL) at 24 weeks after the end of retreatment (72 weeks), was also assessed.
RESULTS
Three male and four female patients were included in the study. The mean (± SD) age was 54.0 ± 10.1 years. Two patients had HCV genotype 1a infection
and five patients had HCV genotype 1b infection. All patients had F3 fibrosis.
Decline in HCV RNA
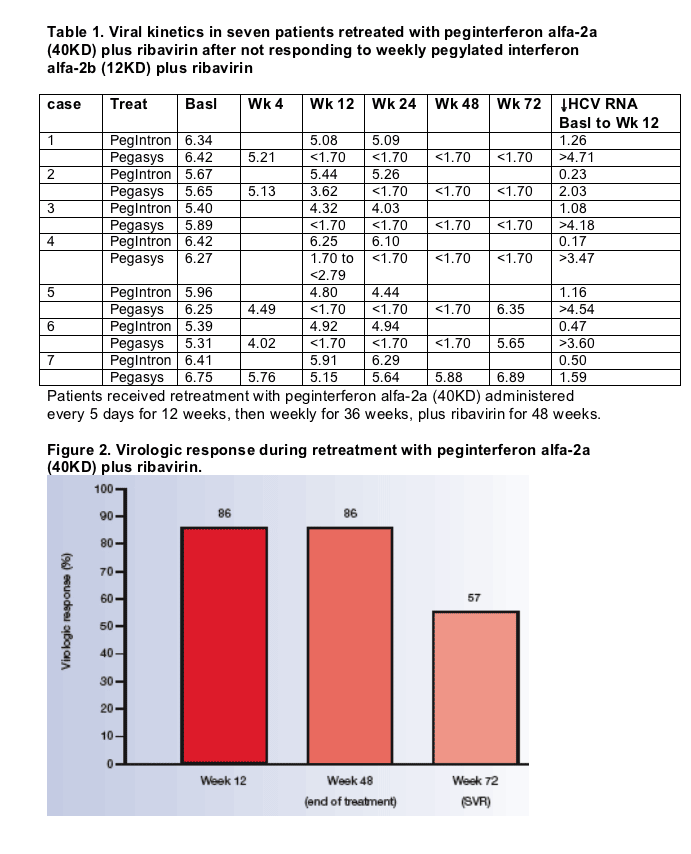
HCV RNA level at weeks 4, 12, 24, 48 and 72 of retreatment, and change in
HCV RNA level from baseline to 12 weeks are shown in Table 1.
- after 12 weeks' retreatment with peginterferon alfa-2a (40KD), HCV RNA had declined by 1.59 to >4.72 log10 IU/mL. In contrast, the decline in HCV RNA with once-weekly pegylated interferon alfa-2b (12KD) (initial treatment) was 0.17 to 1.26 log10 IU/mL
- mean (± SD) HCV RNA decline at week 12 was significantly more pronounced during retreatment with peginterferon alfa-2a (40KD) than during initial treatment with weekly pegylated interferon alfa-2b (12KD) (3.66 ± 1.35 vs 0.70 ± 0.46 log10 IU/mL, respectively) (Figure 1).
Virologic response
Six of seven patients (86%) achieved a virologic response after 12 weeks'retreatment with peginterferon alfa-2a (40KD) plus ribavirin (Figure 2).
- all six patients with a virologic response at 12 weeks also achieved an
end-of-treatment virologic response (Figure 2). Overall, four patients (57%) achieved an SVR (Figure 2).
Safety
The adverse event profile of peginterferon alfa-2a (40KD) administered every 5 days plus ribavirin was similar to that previously reported with peginterferon alfa-2a (40KD) administered once weekly plus ribavirin.[2,3]
- moreover, the tolerability and safety profile of peginterferon alfa-2a (40KD) retreatment was not different from that of pegylated interferon alfa-2b (12KD) during initial treatment.
No decompensation of liver disease was observed and no dose interruptions or treatment discontinuations due to adverse events or laboratory abnormalities were needed.
No patient developed neutropenia (neutrophils <0.5 x 109 cells/L) or thrombocytopenia (platelets <50 x 109 cells/L).
|
| |
|
 |
 |
|
|